Chemical Reactions Physical Science Chapter 7 Ch 7




- Slides: 18

Chemical Reactions Physical Science Chapter 7

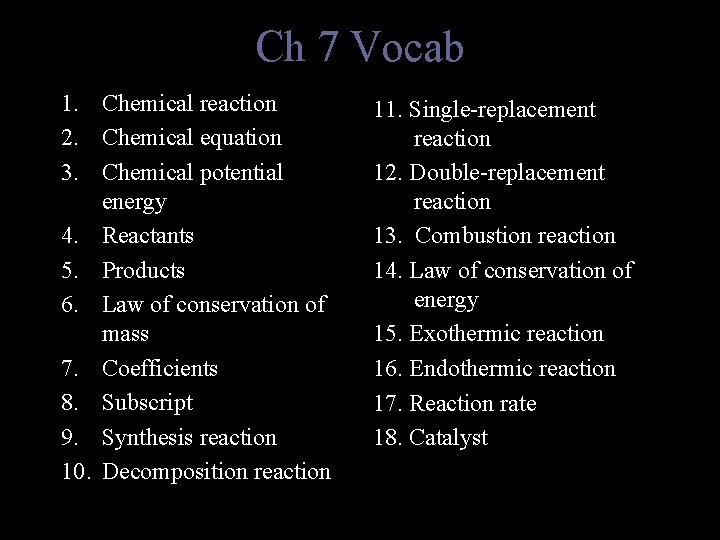
Ch 7 Vocab 1. Chemical reaction 2. Chemical equation 3. Chemical potential energy 4. Reactants 5. Products 6. Law of conservation of mass 7. Coefficients 8. Subscript 9. Synthesis reaction 10. Decomposition reaction 11. Single-replacement reaction 12. Double-replacement reaction 13. Combustion reaction 14. Law of conservation of energy 15. Exothermic reaction 16. Endothermic reaction 17. Reaction rate 18. Catalyst

Ch 7 “I cans…” Law of Conservation of Matter “I Can…” Statements 1 I can identify the meaning of the term “chemical change” as a process that results in the production of a new substance with unique chemical and physical properties. 2 I can balance simple chemical equations applying the conservation of matter. a) I can show that when substances undergo chemical change, the number of atoms in the reactants is the same as the number of atoms in the products. b) I can show that mass is conserved when substances undergo chemical change. 1) I can show that the total mass of the reactants is the same as the total mass of the products. 1 2 3

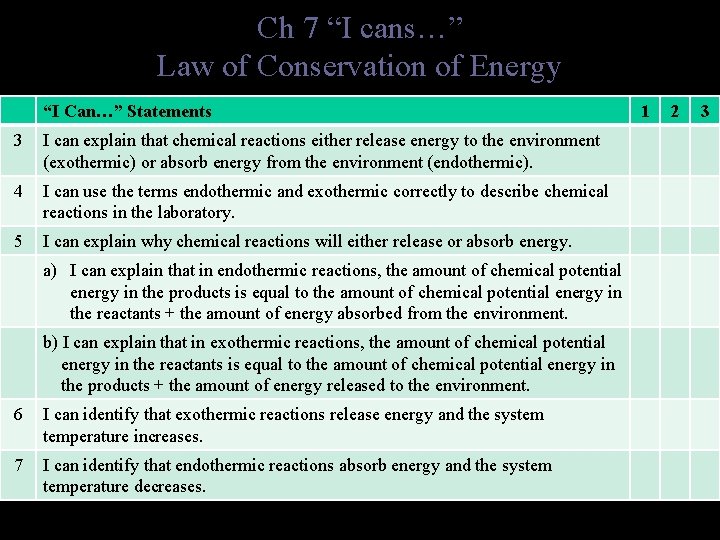
Ch 7 “I cans…” Law of Conservation of Energy “I Can…” Statements 3 I can explain that chemical reactions either release energy to the environment (exothermic) or absorb energy from the environment (endothermic). 4 I can use the terms endothermic and exothermic correctly to describe chemical reactions in the laboratory. 5 I can explain why chemical reactions will either release or absorb energy. a) I can explain that in endothermic reactions, the amount of chemical potential energy in the products is equal to the amount of chemical potential energy in the reactants + the amount of energy absorbed from the environment. b) I can explain that in exothermic reactions, the amount of chemical potential energy in the reactants is equal to the amount of chemical potential energy in the products + the amount of energy released to the environment. 6 I can identify that exothermic reactions release energy and the system temperature increases. 7 I can identify that endothermic reactions absorb energy and the system temperature decreases. 1 2 3

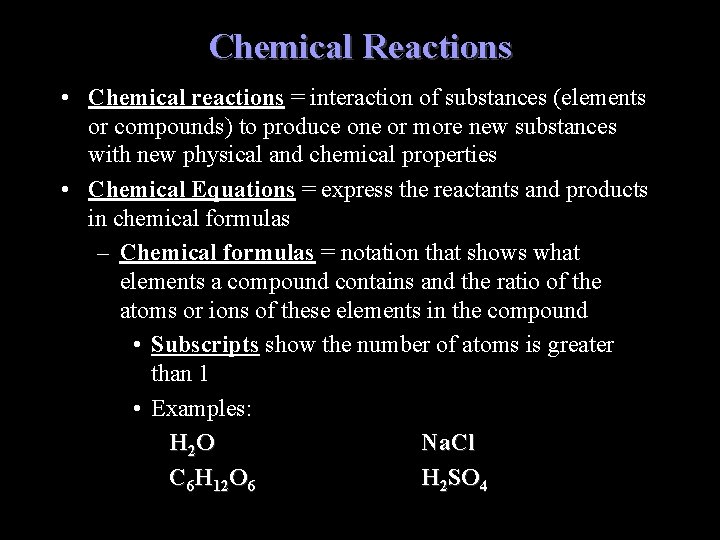
Chemical Reactions • Chemical reactions = interaction of substances (elements or compounds) to produce one or more new substances with new physical and chemical properties • Chemical Equations = express the reactants and products in chemical formulas – Chemical formulas = notation that shows what elements a compound contains and the ratio of the atoms or ions of these elements in the compound • Subscripts show the number of atoms is greater than 1 • Examples: H 2 O Na. Cl C 6 H 12 O 6 H 2 SO 4

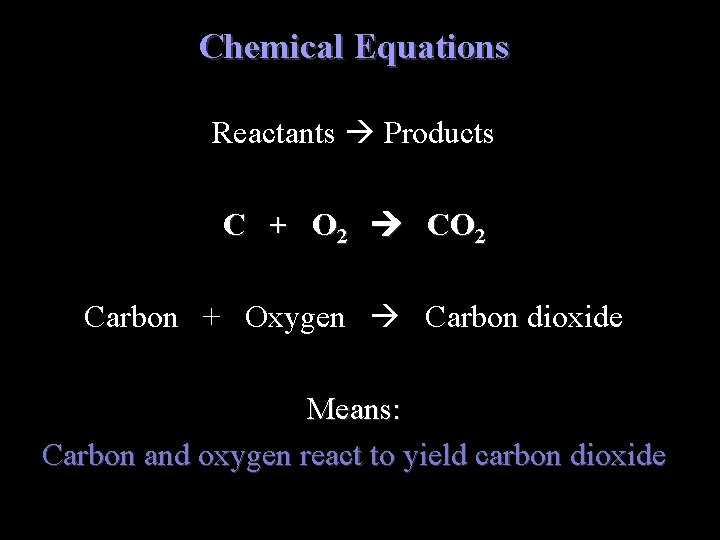
Chemical Equations Reactants Products C + O 2 CO 2 Carbon + Oxygen Carbon dioxide Means: Carbon and oxygen react to yield carbon dioxide

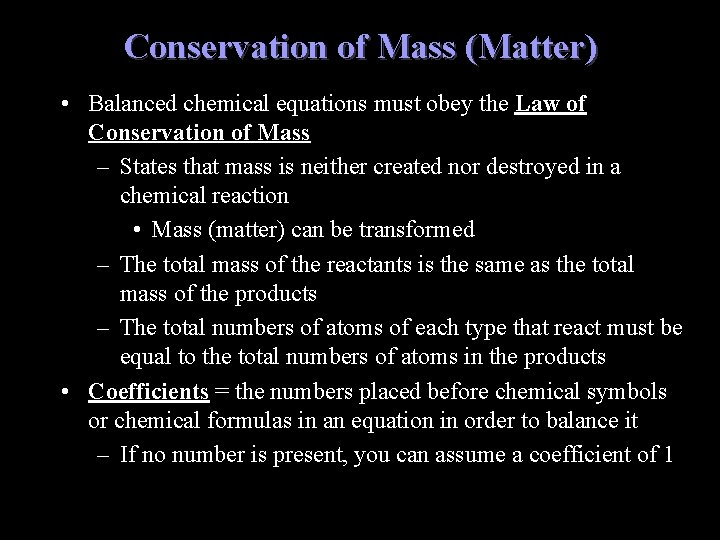
Conservation of Mass (Matter) • Balanced chemical equations must obey the Law of Conservation of Mass – States that mass is neither created nor destroyed in a chemical reaction • Mass (matter) can be transformed – The total mass of the reactants is the same as the total mass of the products – The total numbers of atoms of each type that react must be equal to the total numbers of atoms in the products • Coefficients = the numbers placed before chemical symbols or chemical formulas in an equation in order to balance it – If no number is present, you can assume a coefficient of 1

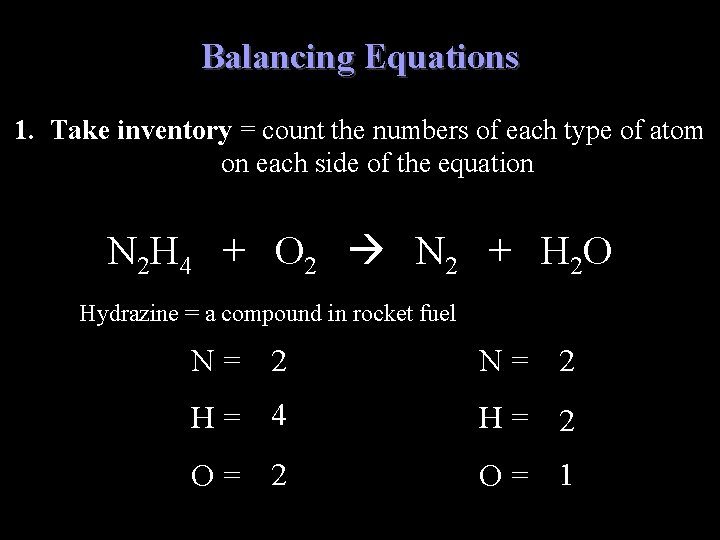
Balancing Equations 1. Take inventory = count the numbers of each type of atom on each side of the equation N 2 H 4 + O 2 N 2 + H 2 O Hydrazine = a compound in rocket fuel N= 2 H= 4 H= 2 O= 1

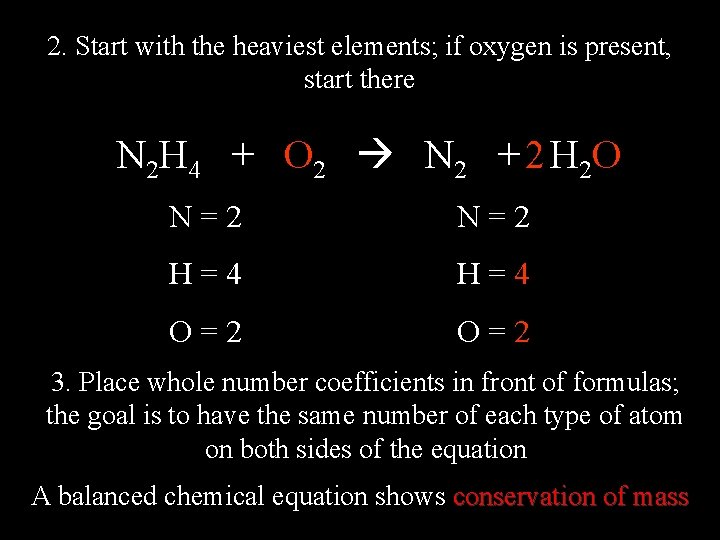
2. Start with the heaviest elements; if oxygen is present, start there N 2 H 4 + O 2 N 2 + 2 H 2 O N=2 H=4 O=2 3. Place whole number coefficients in front of formulas; the goal is to have the same number of each type of atom on both sides of the equation A balanced chemical equation shows conservation of mass

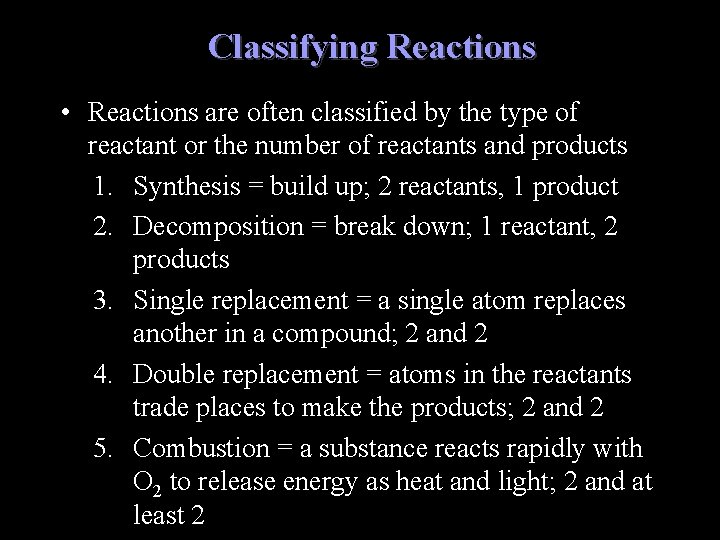
Classifying Reactions • Reactions are often classified by the type of reactant or the number of reactants and products 1. Synthesis = build up; 2 reactants, 1 product 2. Decomposition = break down; 1 reactant, 2 products 3. Single replacement = a single atom replaces another in a compound; 2 and 2 4. Double replacement = atoms in the reactants trade places to make the products; 2 and 2 5. Combustion = a substance reacts rapidly with O 2 to release energy as heat and light; 2 and at least 2

C 3. 4 Endothermic and Exothermic Reactions I can explain that chemical interactions either release energy to the environment (exothermic) or absorb energy from the environment (endothermic). C 3. 4 A I can use the terms endothermic and exothermic correctly to describe chemical reactions in the laboratory. C 3. 4 B I can explain why chemical reactions will either release or absorb energy.

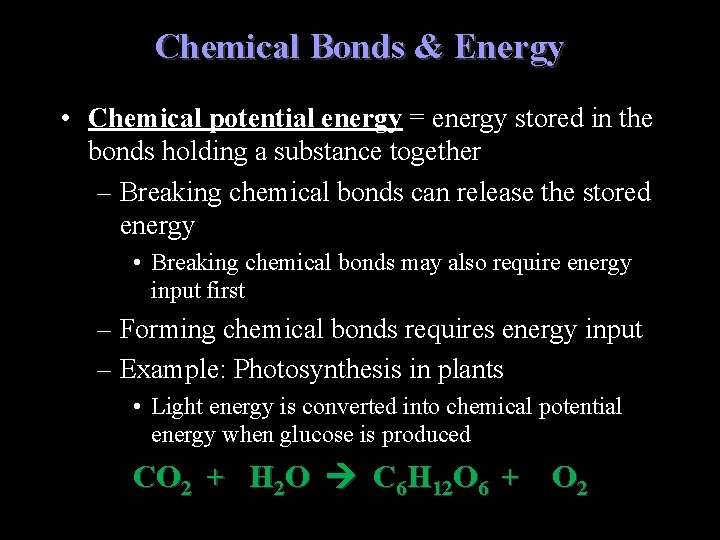
Chemical Bonds & Energy • Chemical potential energy = energy stored in the bonds holding a substance together – Breaking chemical bonds can release the stored energy • Breaking chemical bonds may also require energy input first – Forming chemical bonds requires energy input – Example: Photosynthesis in plants • Light energy is converted into chemical potential energy when glucose is produced CO 2 + H 2 O C 6 H 12 O 6 + O 2

Law of Conservation of Energy • States that energy can neither be created nor destroyed – Energy can be transformed • Energy is conserved in chemical reactions – The energy in the products must be equal to the energy in the reactants because energy is either absorbed or released

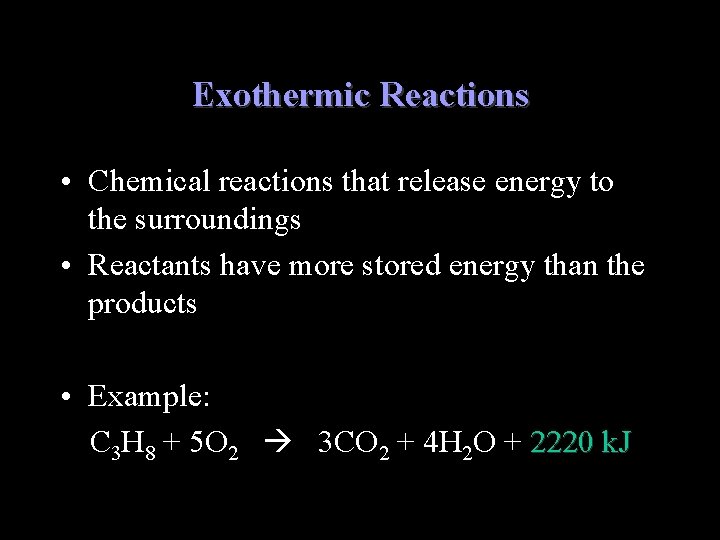
Exothermic Reactions • Chemical reactions that release energy to the surroundings • Reactants have more stored energy than the products • Example: C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O + 2220 k. J

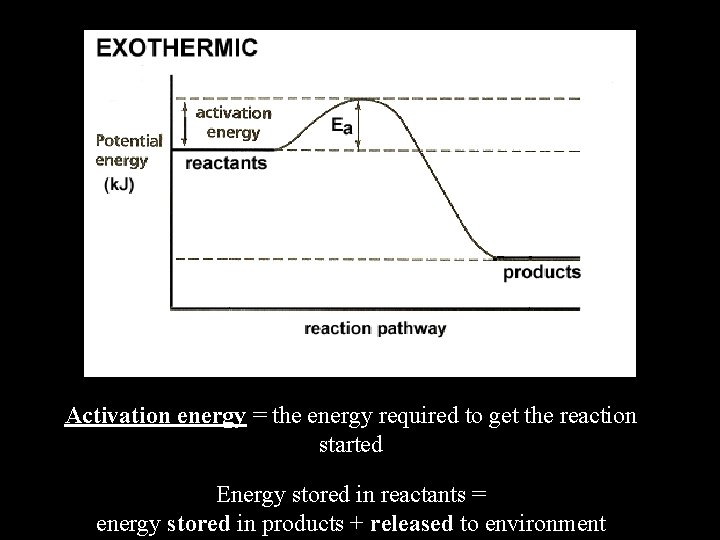
Activation energy = the energy required to get the reaction started Energy stored in reactants = energy stored in products + released to environment

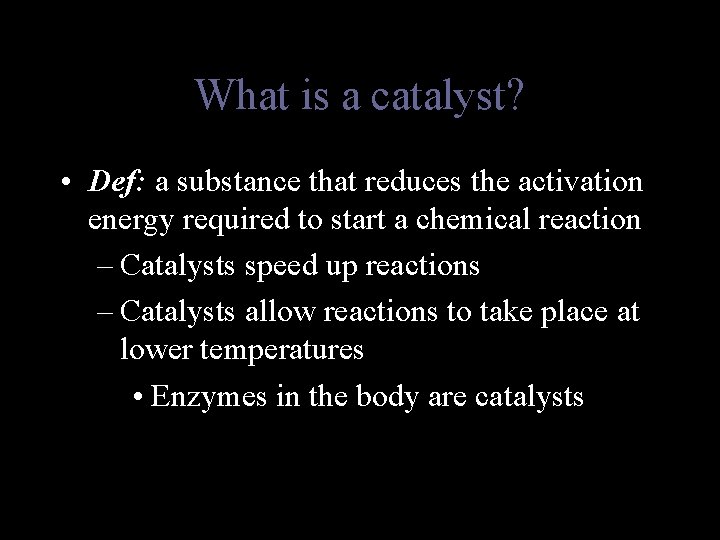
What is a catalyst? • Def: a substance that reduces the activation energy required to start a chemical reaction – Catalysts speed up reactions – Catalysts allow reactions to take place at lower temperatures • Enzymes in the body are catalysts

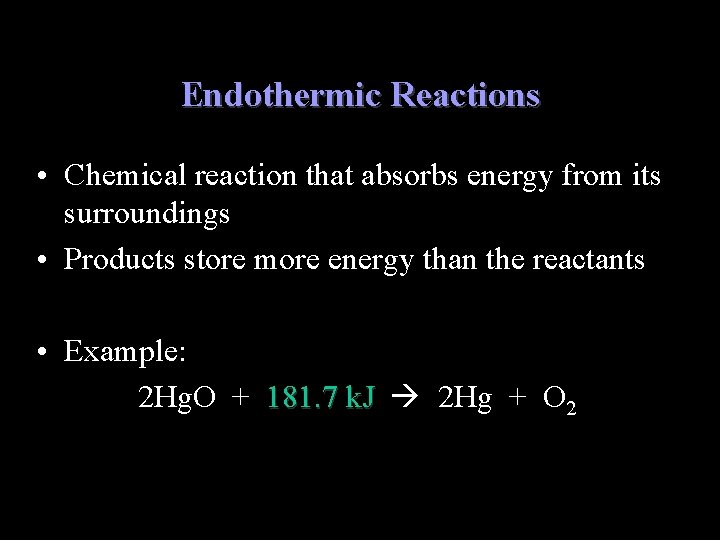
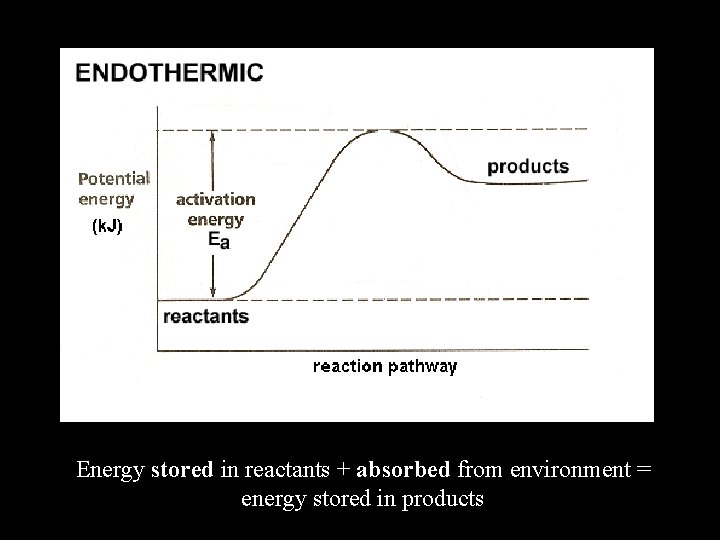
Endothermic Reactions • Chemical reaction that absorbs energy from its surroundings • Products store more energy than the reactants • Example: 2 Hg. O + 181. 7 k. J 2 Hg + O 2

Energy stored in reactants + absorbed from environment = energy stored in products