CHEMICAL REACTIONS Occur when substances undergo chemical changes
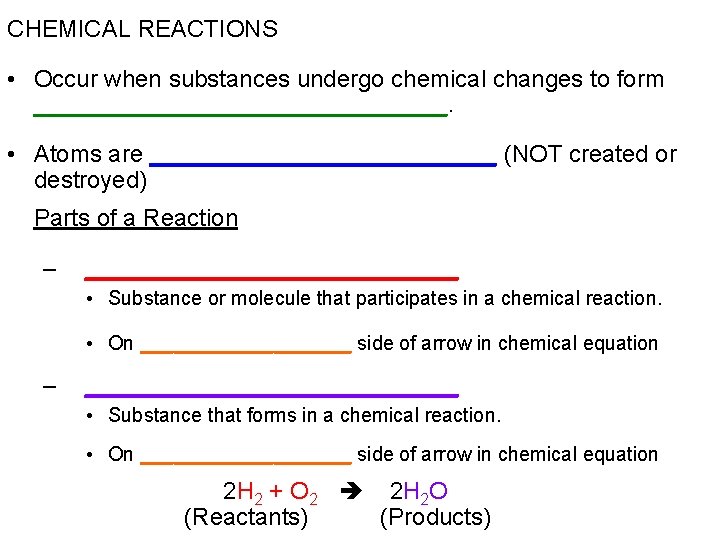
CHEMICAL REACTIONS • Occur when substances undergo chemical changes to form ________________. • Atoms are _____________ (NOT created or destroyed) Parts of a Reaction – ______________ • Substance or molecule that participates in a chemical reaction. • On __________ side of arrow in chemical equation – ______________ • Substance that forms in a chemical reaction. • On __________ side of arrow in chemical equation 2 H 2 + O 2 2 H 2 O (Reactants) (Products)
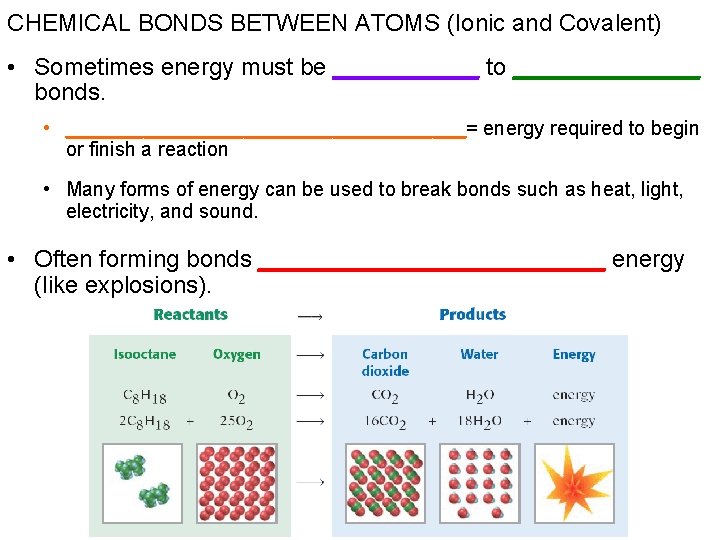
CHEMICAL BONDS BETWEEN ATOMS (Ionic and Covalent) • Sometimes energy must be ______ to _______ bonds. • __________________= energy required to begin or finish a reaction • Many forms of energy can be used to break bonds such as heat, light, electricity, and sound. • Often forming bonds _____________ energy (like explosions).
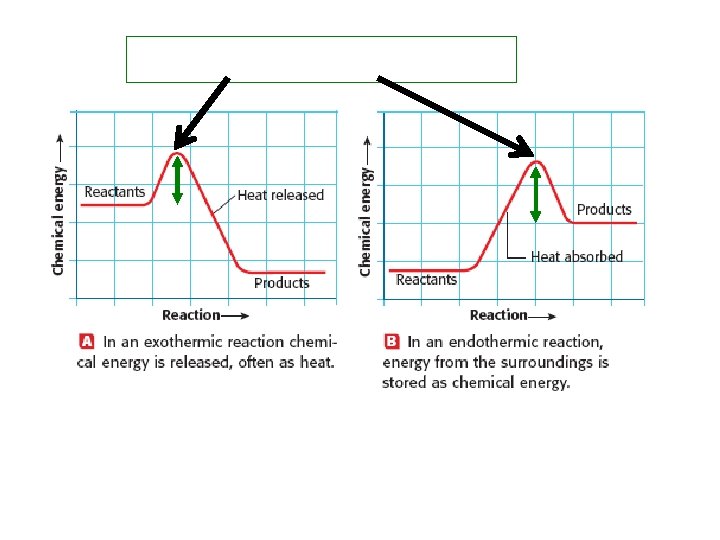
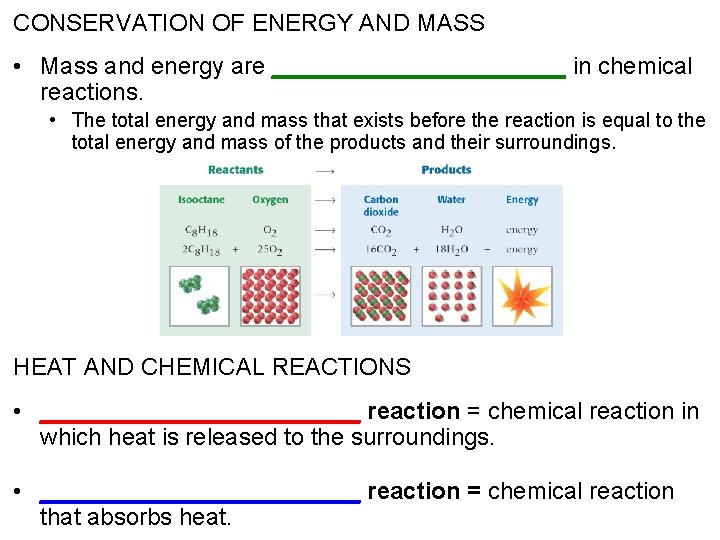
CONSERVATION OF ENERGY AND MASS • Mass and energy are ___________ in chemical reactions. • The total energy and mass that exists before the reaction is equal to the total energy and mass of the products and their surroundings. HEAT AND CHEMICAL REACTIONS • ____________ reaction = chemical reaction in which heat is released to the surroundings. • ____________ reaction = chemical reaction that absorbs heat.

SPEED OF A REACTION • _____________ = substance that changes the _______________ without being consumed or changed significantly. • Catalysts are _______ reactants or products, because they are not used up in the reaction. • Catalysts are often used in industry to make reactions go faster. • Catalysts in your body are called _____________
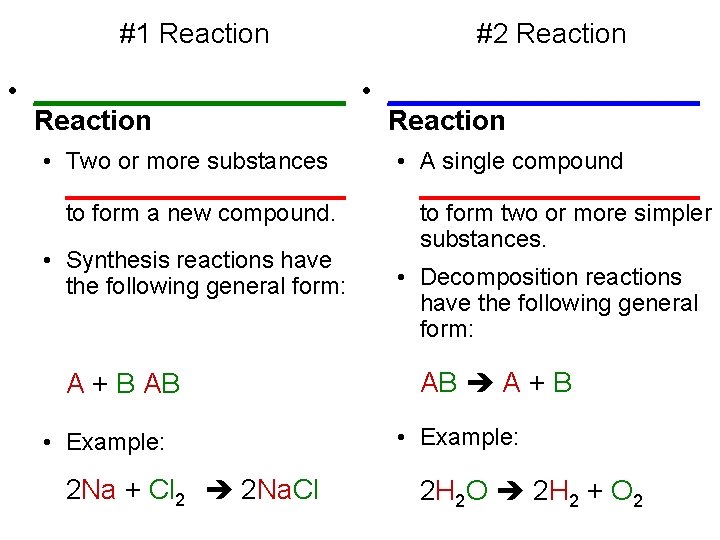
#1 Reaction #2 Reaction • ____________________ Reaction • Two or more substances ___________ to form a new compound. • Synthesis reactions have the following general form: A + B AB • Example: 2 Na + Cl 22 2 Na. Cl • A single compound ___________ to form two or more simpler substances. • Decomposition reactions have the following general form: AB A + B • Example: 2 H 2 O 2 H 2 + O 2

# 3 Reaction #4 Reaction • ________________________ Reaction • ______ element or ion _____________ of another element or ion in the compound. • Single-displacement reactions have the following general form: AX + B BX + A • Example: 3 Cu. Cl 2 + 2 Al. Cl 3 + 3 Cu • A gas, a solid precipitate, or a molecular compound forms from the apparent __________________________ between _______ compounds. • Double-displacement reactions have the following general form: AX + BY AY + BX • Example: Pb(NO 3)2 + K 2 Cr. O 4 Pb. Cr. O 4 + 2 KNO 3
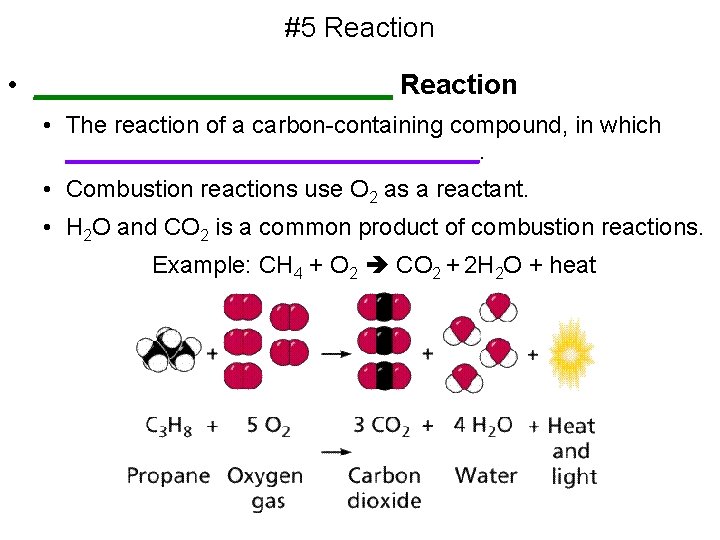
#5 Reaction • ____________ Reaction • The reaction of a carbon-containing compound, in which ________________. • Combustion reactions use O 2 as a reactant. • H 2 O and CO 2 is a common product of combustion reactions. Example: CH 4 + O 2 CO 2 + 2 H 2 O + heat
- Slides: 8