Chemical Reactions Objectives 1 Write a word equation
Chemical Reactions Objectives: 1. Write a word equation 2. Write a skeletal equations 3. Describe the parts to a chemical reaction
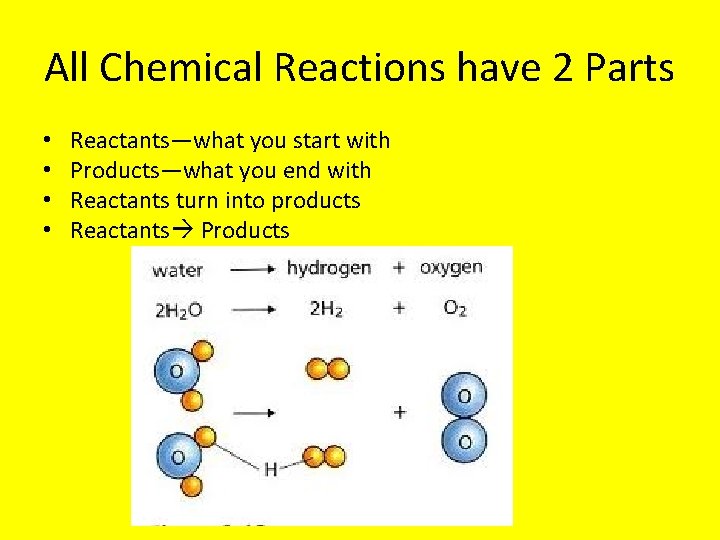
All Chemical Reactions have 2 Parts • • Reactants—what you start with Products—what you end with Reactants turn into products Reactants Products

Law of Conservation of Mass • Atoms aren’t created nor destroyed, just rearranged • Can be expressed in a few ways: • Sentence: – Copper reacts with chlorine to form copper(II) chloride • Word equation – Copper + chlorine copper(II) chloride • Skeletal – Cu + Cl Cu. Cl

Symbols • Arrow ( ) separates reactants from products (points to products) – Read as “reacts to form” or “yields” • • • +-- “and” (s) after formula—solid (g) after formula= gas (l) after formula=liquid (aq)—dissolved in water—aqueous solution Double arrow—reversible reaction

More Symbols or with “heat” over arrow—heat is used in the reaction • Arrow with “catalyst” or “enzyme” above it means a catalyst or enzyme was used. Sometimes they will use the actual enzyme. •
Catalyst • Substance that speeds up a reaction, without being changed or used up by the reaction. • Enzymes are biological catalysts in your body
Skeletal Equations • Uses formulas and symbols to describe a reaction – Does not indicate how many, they are NOT balanced

Writing Skeletal Equations • Solid Iron (III) sulfide reacts with gaseous hydrogen chloride to form iron (III) chloride and hydrogen sulfide gas.
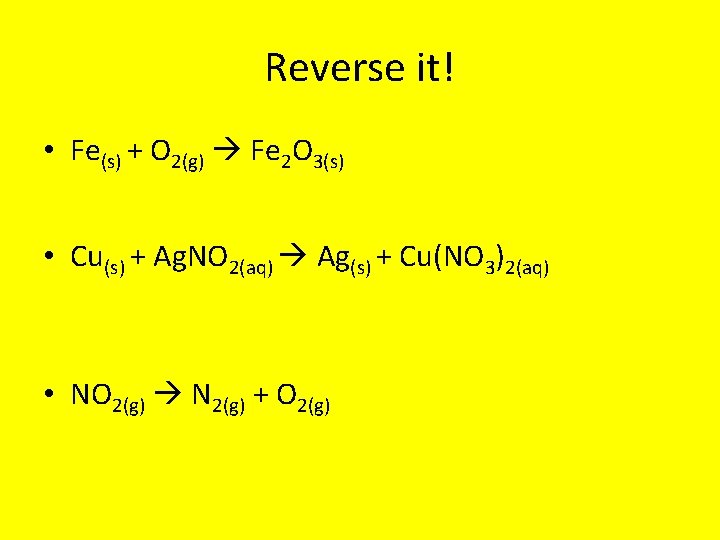
Reverse it! • Fe(s) + O 2(g) Fe 2 O 3(s) • Cu(s) + Ag. NO 2(aq) Ag(s) + Cu(NO 3)2(aq) • NO 2(g) N 2(g) + O 2(g)

Balancing Chemical Equations • Atoms can’t be created or destroyed – All the atoms we start with we must end up with • A balanced equation has the same number of each element on both sides of the equation

Rules • Assemble the correct formulas for all the reactants and products • Count the number of atoms of each type appearing on both sides • Balance the elements one at a time by adding coefficients (number in the front). • Balance Hydrogen and Oxygen last! • Double check
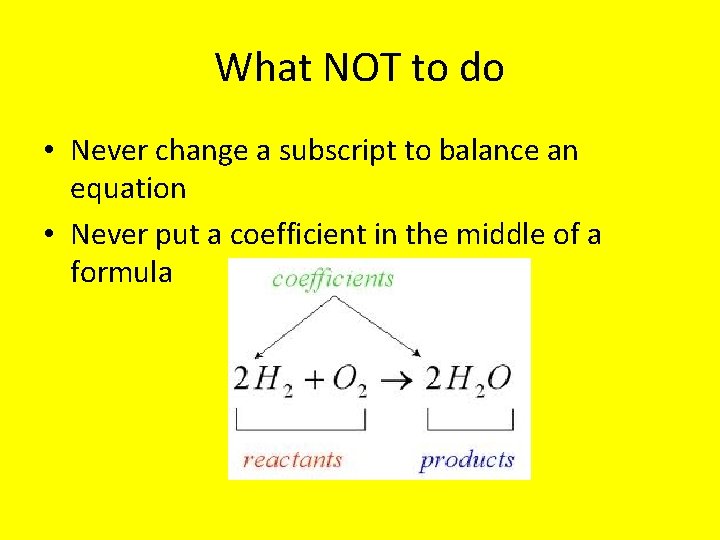
What NOT to do • Never change a subscript to balance an equation • Never put a coefficient in the middle of a formula

Example
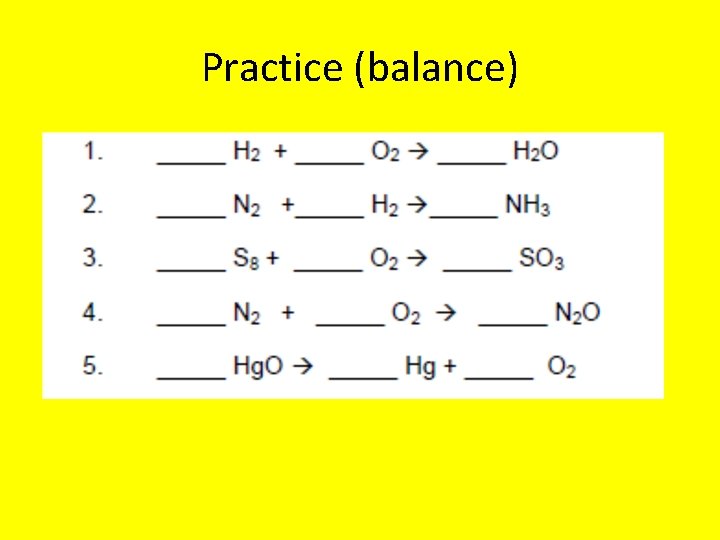
Practice (balance)
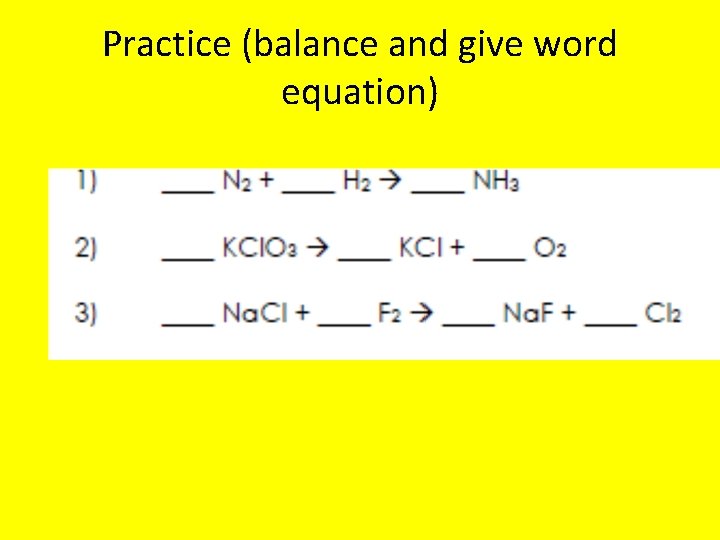
Practice (balance and give word equation)

Give the chemical equation and then balance • Zinc and lead (II) nitrate react to form zinc nitrate and lead. • Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas • Sodium phosphate and calcium chloride react to form calcium phosphate and sodium chloride
- Slides: 16