Chemical Reactions Ms Gacke Chem Catalyst Chemical Reactions

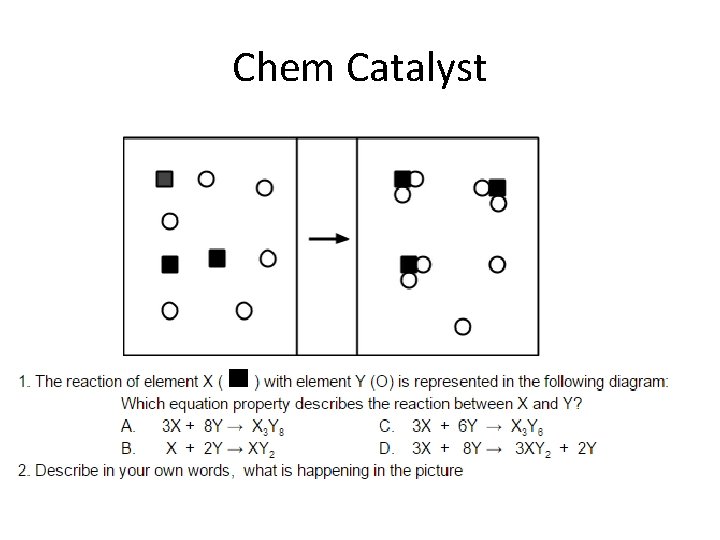










- Slides: 18

Chemical Reactions Ms. Gacke

Chem Catalyst

Chemical Reactions • Synthesis Reaction: When two substances combine and form a compound. • Reactant + Reactant 1 product A + B AB

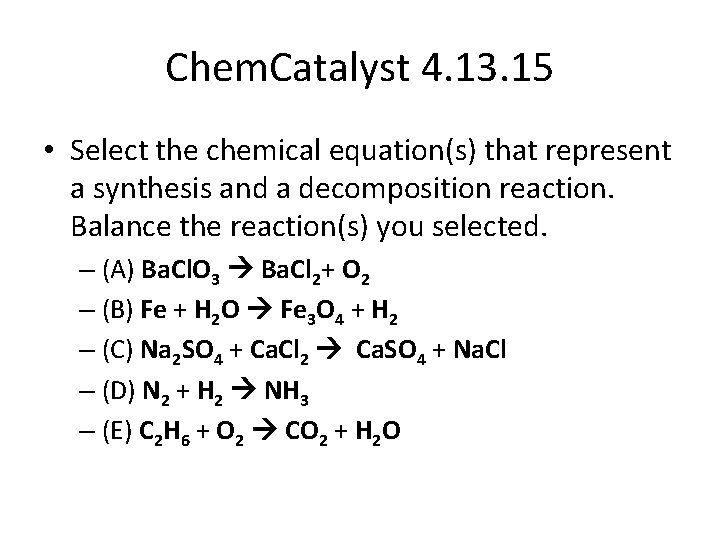
Chem. Catalyst 4. 13. 15 • Select the chemical equation(s) that represent a synthesis and a decomposition reaction. Balance the reaction(s) you selected. – (A) Ba. Cl. O 3 Ba. Cl 2+ O 2 – (B) Fe + H 2 O Fe 3 O 4 + H 2 – (C) Na 2 SO 4 + Ca. Cl 2 Ca. SO 4 + Na. Cl – (D) N 2 + H 2 NH 3 – (E) C 2 H 6 + O 2 CO 2 + H 2 O

Practice Predict the products. Write and balance the following synthesis equations on your whiteboards 1. Na(s) + Cl 2(g) 2. Mg(s) + F 2(g) 3. Aluminum metal reacts with Fluorine gas

Decomposition Reactions • When a compound breaks down into simpler compounds or elements • 1 Reactant product + product AB A + B Catalyst: to speed up the reaction -chemical (compound name will appear over the ) -energy/heat (triangle over the )

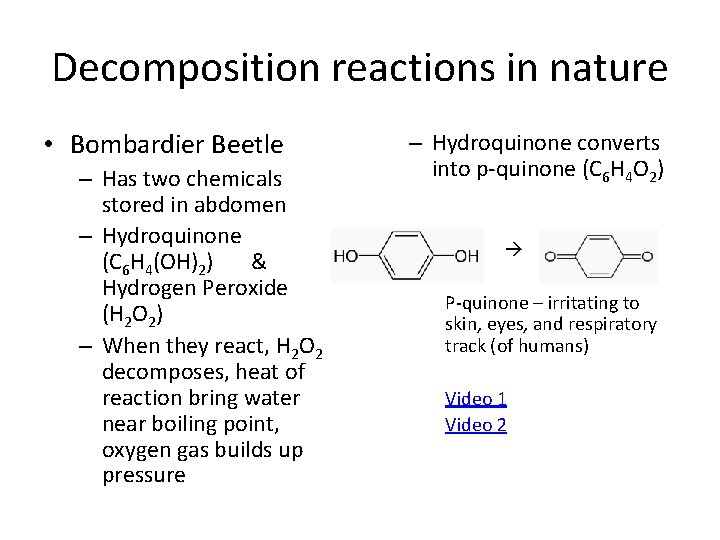
Decomposition reactions in nature • Bombardier Beetle – Has two chemicals stored in abdomen – Hydroquinone (C 6 H 4(OH)2) & Hydrogen Peroxide (H 2 O 2) – When they react, H 2 O 2 decomposes, heat of reaction bring water near boiling point, oxygen gas builds up pressure – Hydroquinone converts into p-quinone (C 6 H 4 O 2) P-quinone – irritating to skin, eyes, and respiratory track (of humans) Video 1 Video 2

Decomp Practice • Predict the products of the following decomposition reactions • 1. KCl. O 3 ____ + ____ • 2. Mg. Cl 2 ____ + ____ • 3. Ba(Cl. O 3)2 ____ + ____

Synthesis/Decomposition Review • On your white board – Draw a picture explaining synthesis reactions that is not related to chemistry. – Draw a picture explaining decomposition reactions that is not related to chemistry

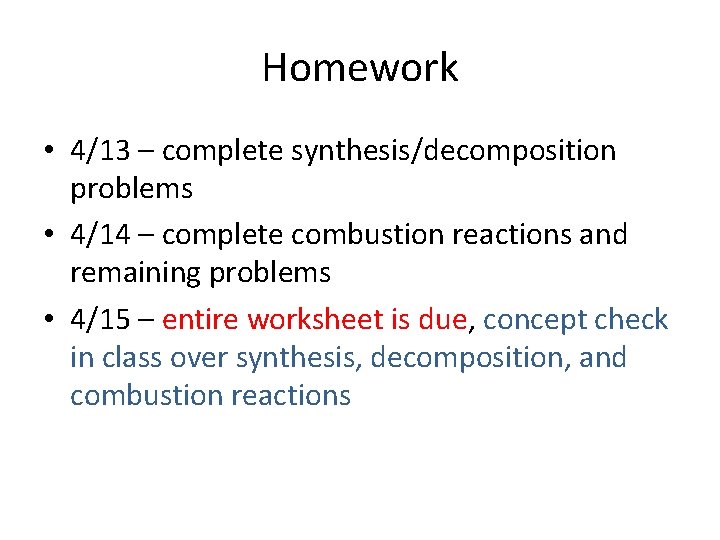
Homework • 4/13 – complete synthesis/decomposition problems • 4/14 – complete combustion reactions and remaining problems • 4/15 – entire worksheet is due, concept check in class over synthesis, decomposition, and combustion reactions

Chem. Catalyst 4/14/15 • Watch the demonstration • Answer the following question: – What evidence do you have that Mr. Hall is or is not crazy?

What are the requirements for combustion? • Reactants: • Products:

Combustion Reaction • Hydrocarbon + O 2 CO 2 + H 2 O • What is a hydrocarbon? • Common examples of hydrocarbons?

Remainder of class time to work on HW and get assistance 4/13 – complete synthesis/decomposition problems 4/14 – complete combustion reactions and remaining problems 4/15 – entire worksheet is due, concept check in class over synthesis, decomposition, and combustion reactions

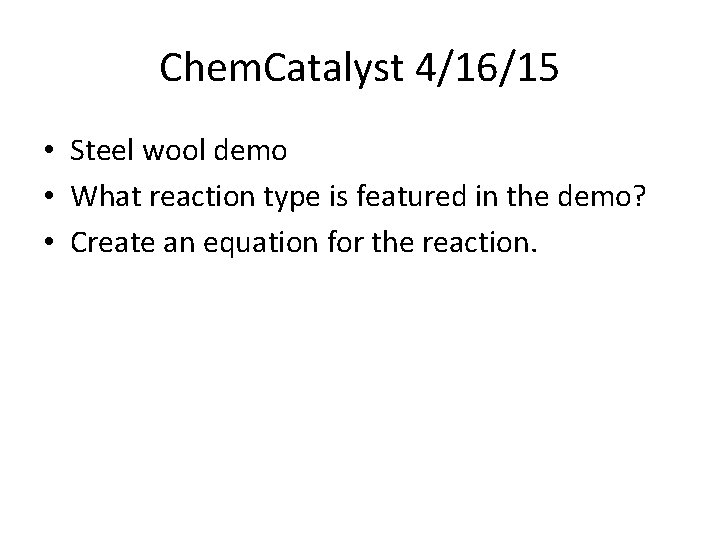
Chem. Catalyst 4/16/15 • Steel wool demo • What reaction type is featured in the demo? • Create an equation for the reaction.

Single Replacement Reaction Demo – Copy into your notes! • • • Single Replacement: Demo: Aluminum and Copper (II) Chloride Indication of a chemical change: Possible Products: Balanced Reaction:

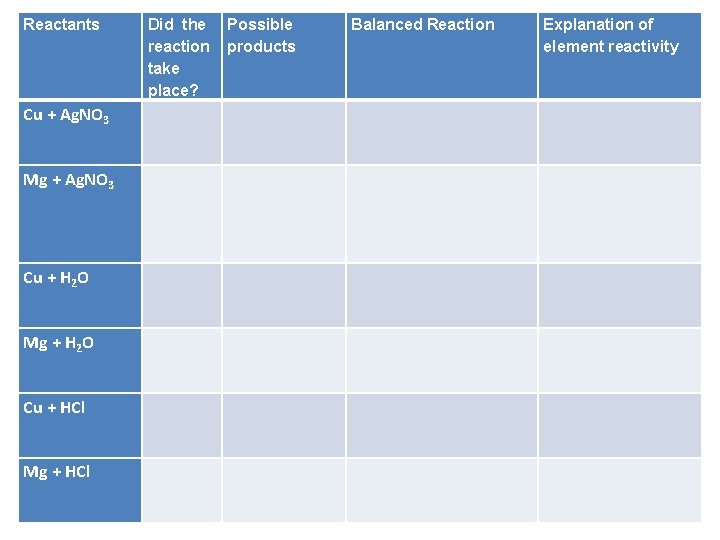
Reactants Did the reaction take place? Possible products Balanced Reaction Explanation of element reactivity Cu + Ag. NO 3 Mg + Ag. NO 3 Cu + H 2 O Mg + H 2 O Cu + HCl Mg + HCl

 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Oxidation half reaction
Oxidation half reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Chemical reaction of bread
Chemical reaction of bread Site do professor
Site do professor Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Solvent in chemical reactions
Solvent in chemical reactions Chemical reactions study guide
Chemical reactions study guide Understanding chemical reactions worksheet answer key
Understanding chemical reactions worksheet answer key What are the five chemical changes
What are the five chemical changes How to identify types of chemical reactions
How to identify types of chemical reactions Percent yield of copper
Percent yield of copper Unit 11 chemical reactions
Unit 11 chemical reactions What is an active metal
What is an active metal



































