CHEMICAL REACTIONS MATTER IS ALWAYS CHANGING Physical change






























- Slides: 40

CHEMICAL REACTIONS

MATTER IS ALWAYS CHANGING • Physical change – the chemical composition of matter does not change • Chemical change – chemical composition of matter is not constant • Chemical reaction- When a substance undergoes a chemical change and forms a new substance

IF YOU MIX TWO THINGS TOGETHER HOW DO YOU KNOW A CHEMICAL REACTION HAS OCCURRED?

INDICATORS THAT A CHEMICAL CHANGE HAS OCCURRED • Gas produced (bubbles) • Energy change • Exothermic • Endothermic • Light • Precipitate is formed – precipitate is an insoluble solid formed from combining two aqueous solutions • Permanent color change • p. H change

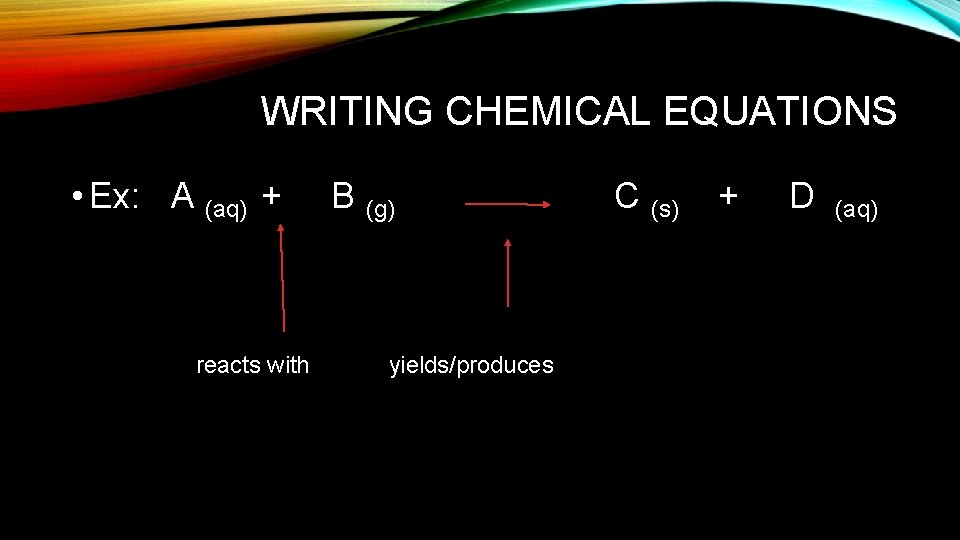
WRITING CHEMICAL EQUATIONS • Reactants products • Reactants – what you start with • Products – new substances formed

SYMBOLS FOR REACTIONS • • • + separates 2 reactants or 2 products (s) solid (l) liquid (g) gas (aq) aqueous – solid dissolved in a liquid usually water (c) crystal precipitate produced gas produced Δ energy is needed yields or produces- separates reactants from products

WRITING CHEMICAL EQUATIONS • Ex: A (aq) + reacts with B (g) yields/produces C (s) + D (aq)

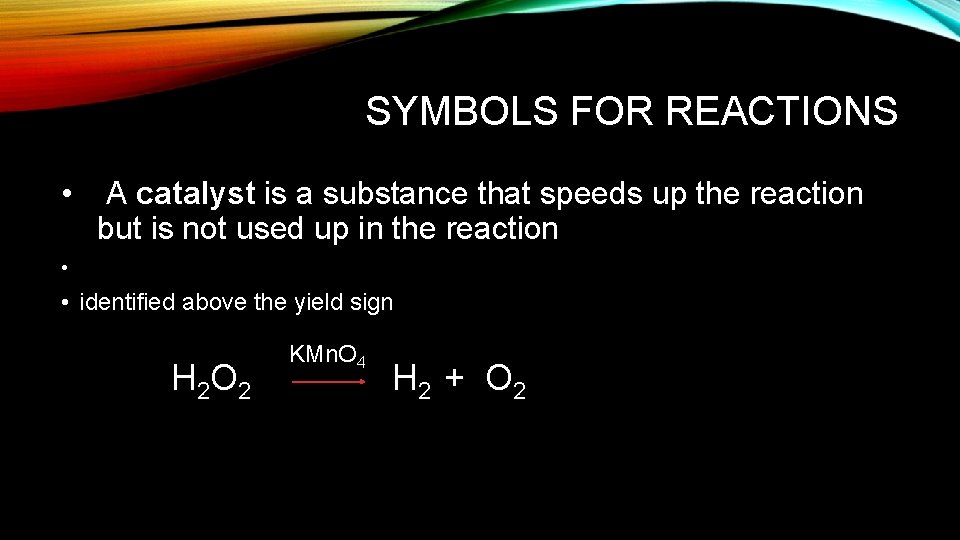
SYMBOLS FOR REACTIONS • A catalyst is a substance that speeds up the reaction but is not used up in the reaction • • identified above the yield sign H 2 O 2 KMn. O 4 H 2 + O 2

PRACTICE WRITING CHEMICAL EQUATIONS • Write the chemical equation for the following reactions 1. Zinc metal reacts with hydrogen chloride to produce aqueous zinc chloride and hydrogen gas 2. Aqueous solutions of sodium iodide and silver nitrate yield silver iodide precipitate and aqueous sodium nitrate

BALANCING CHEMICAL EQUATIONS • Remember the Law of Conservation of Matter/Energy? • We need to balance the equations in order to satisfy this……

EXAMPLE : BUILDING A BICYCLE

11. 1 BALANCING CHEMICAL EQUATIONS • To write a balanced chemical equation, first write the skeleton equation. Then use coefficients to balance the equation so that it obeys the law of conservation of mass.

RULES FOR BALANCING EQUATIONS: • Write correct skeleton formula • Determine number of atoms of each element of reactants and products. COUNT POLYATOMIC ION AS A SINGLE UNIT if it appears unchanged on both sides of the equation • Balance elements one at a time by using coefficients-NEVER change subscripts • Begin with the easiest elements first • Check both sides to see if they match • Make sure coefficients are in the lowest possible ratio

REMEMBER DIATOMICS!! • When you write the skeleton equation, remember these elements must be written as 2 atoms when they are not involved in a compound…. • Br 2 I 2 N 2 Cl 2 H 2 O 2 F 2


TYPES OF REACTIONS

TYPES OF CHEMICAL REACTIONS • Synthesis (combination) • Decomposition • Single Replacement • Double Replacement • Combustion

1. SYNTHESIS • Synthesis (composition, combination) reaction • two or more substances react to form a single new substance. A + B AB

PRACTICE • Predict the products. Write and balance the following synthesis reaction equations. • Sodium metal reacts with chlorine gas Na(s) + Cl 2(g) • Solid Magnesium reacts with fluorine gas Mg(s) + F 2(g)

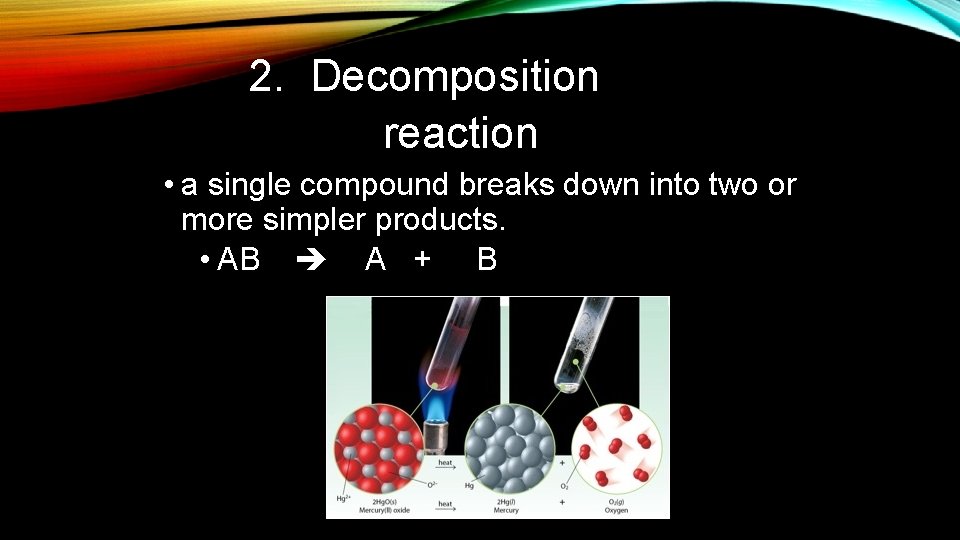
2. Decomposition reaction • a single compound breaks down into two or more simpler products. • AB A + B

2. Decomposition DECOMPOSITION Rules for Reactions: REACTIONS • All binary compounds (ex: Mg. Cl 2) will break down into their elements • All carbonates (CO 32 -) break down into oxide and carbon dioxide • Chlorates (Cl. O 3 -) break down into binary salt and oxygen • Bases (OH group) and compounds with an H and an O will break down into water and an oxide

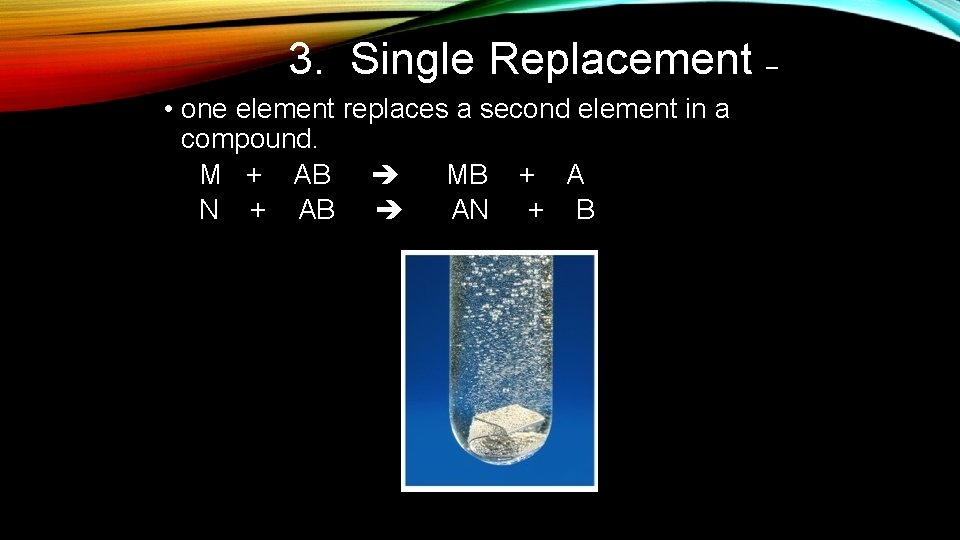
3. Single Replacement – • one element replaces a second element in a compound. M + AB MB + A N + AB AN + B

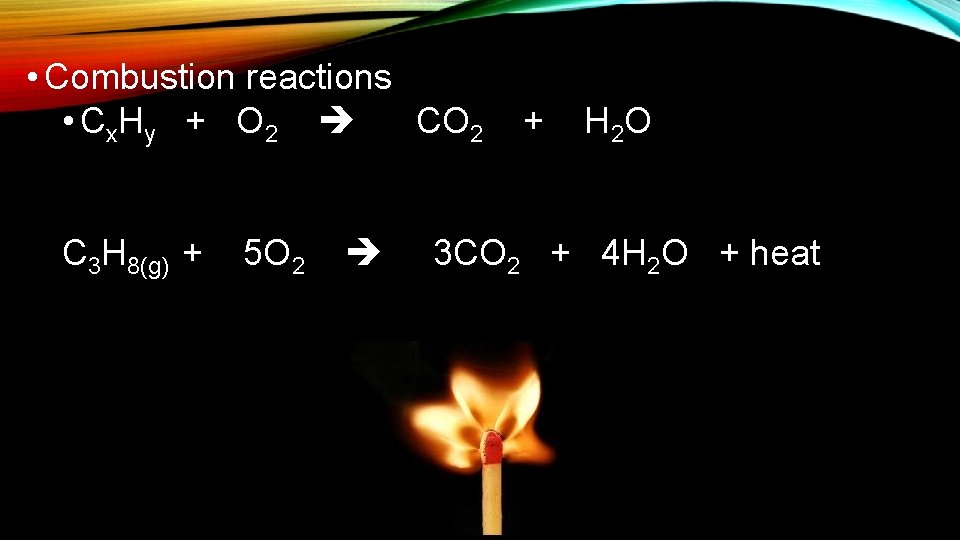
4. COMBUSTION REACTIONS • Combustion reactions occur when a fuel reacts with oxygen gas, which produces heat! Fuel + O 2 (+ Heat) Product

• Combustion reactions • Cx. Hy + O 2 CO 2 C 3 H 8(g) + 5 O 2 + H 2 O 3 CO 2 + 4 H 2 O + heat

5. DOUBLE REPLACEMENT (PRECIPITATION) • Double replacement- also known as precipitation reaction, (and sometimes neutralization reaction) AB + CD AD + CB

DOUBLE REPLACEMENT • If a double replacement reaction involves an acid and a base, then the double replacement reaction is known as a NEUTRALIZATION reaction • Acid + base salt + water • How do you know if it’s an acid and a base? ?

ACIDS AND BASES • Acids – release hydrogen ions (H+) in water • Ex: HCl hydrochloric acid • Bases – releases hydroxide (OH-) in water • Ex: Na. OH sodium hydroxide

SO IN A NEUTRALIZATION REACTION… • HCl + Na. OH Remember that this is a type of double replacement

PRACTICE sulfuric acid neutralizes a solution of potassium hydroxide • 1. • UH OH! What is the formula for sulfuric acid? • Hope you studied your polyatomics !

NAMING ACIDS AND BASES • Bases • Metal keeps its name, hydroxide • Ex : Na. OH • sodium hydroxide

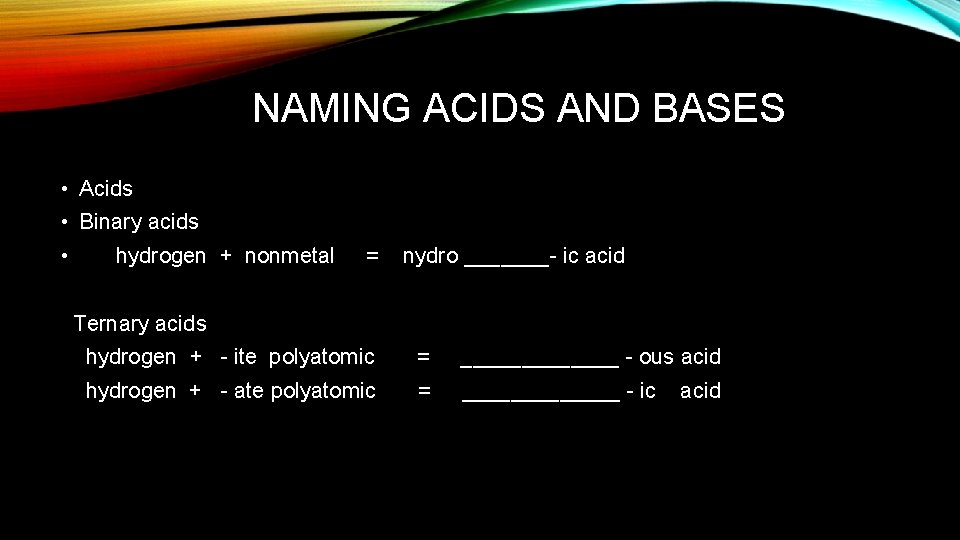
NAMING ACIDS AND BASES • Acids • Binary acids • hydrogen + nonmetal = nydro _______- ic acid Ternary acids hydrogen + - ite polyatomic = _______ - ous acid hydrogen + - ate polyatomic = _______ - ic acid

NAMING ACIDS AND BASES • Acids • Ex: what is the formula for sulfuric acid ? • Ex: what is the formula for nitrous acid ?

SOME PROPERTIES OF ACIDS þ Produce H + (as H 3 O +) ions in water (the hydronium ion is a hydrogen ion attached to a water molecule) þ Taste sour þ Corrode metals þ Electrolytes þ p. H is less than 7 þ Litmus “ends up “ red ( blue turns red, red stays red)

SOME COMMON BASES Na. OH sodium hydroxide KOH potassium hydroxide Ba(OH) 2 barium hydroxide Mg(OH) 2 magnesium hydroxide Al(OH)3 aluminum hydroxide

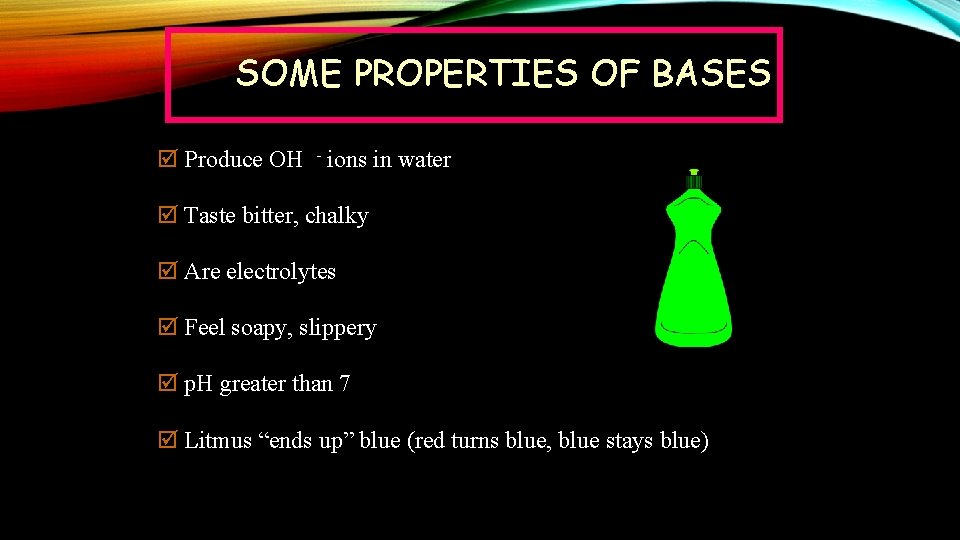
SOME PROPERTIES OF BASES þ Produce OH - ions in water þ Taste bitter, chalky þ Are electrolytes þ Feel soapy, slippery þ p. H greater than 7 þ Litmus “ends up” blue (red turns blue, blue stays blue)

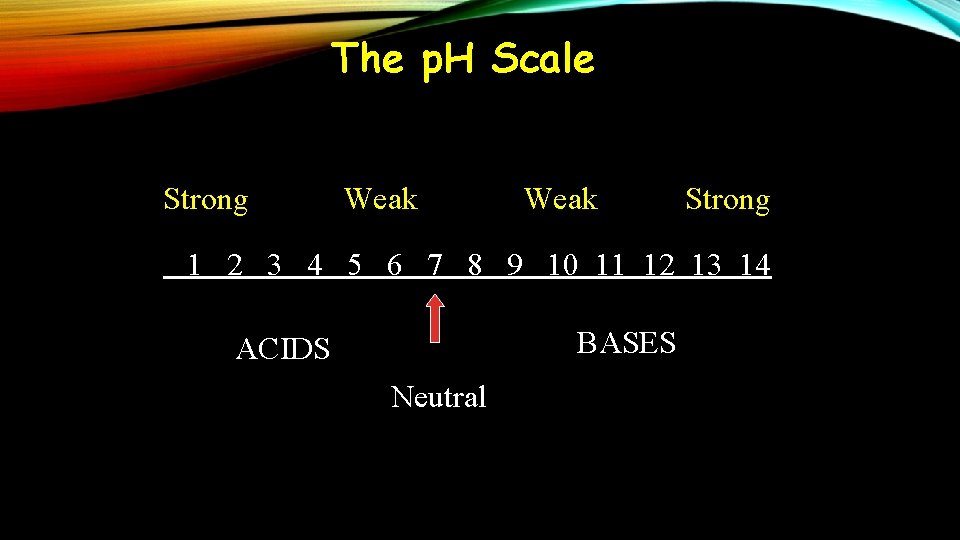
The p. H Scale Strong Weak Strong 1 2 3 4 5 6 7 8 9 10 11 12 13 14 BASES ACIDS Neutral

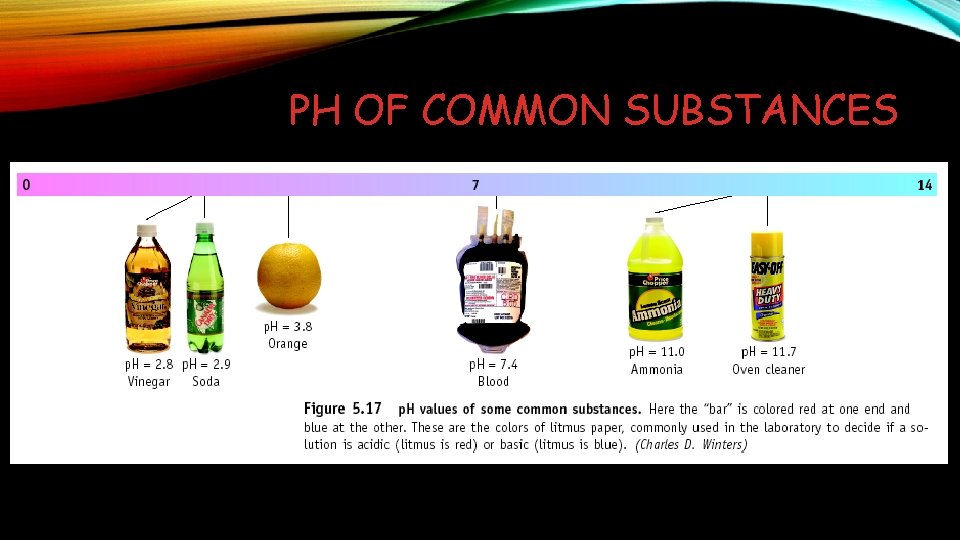
PH OF COMMON SUBSTANCES

WHAT IS PH? • “p” in p. H stands for either potential or power • If more H 3 O + in solution than OH- it’s an acid • If more OH- than H 3 O + then it’s a base • If equal amounts of H 3 O + and OH- then it’s neutral


BUFFER • Buffer is a solution that resists changes and can control p. H when an acid or base is added. • a buffer consumes excess hydrogen ions or hydroxide ions which determine the p. H • Made of aqueous weak acid and one of its salts • Ex: hydrogen carbonate and carbonate ions – controls the p. H of blood at a p. H of 7. 4