Chemical Reactions Involving Oxygen 1 A chemical change



- Slides: 9

Chemical Reactions Involving Oxygen 1. A chemical change is any change in which a new substance is formed. Evidence: Release of energy as heat and light, change in colour, formation of gas, change in odour, etc. 2. sulfur + oxygen sulfur dioxide phosphorus + oxygen diphosphorus pentoxide magnesium + oxygen magnesium oxide iron + oxygen iron(III) oxide

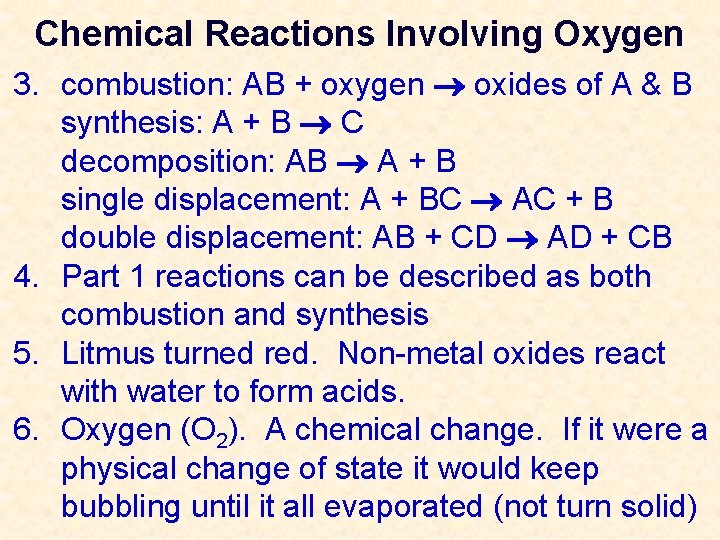
Chemical Reactions Involving Oxygen 3. combustion: AB + oxygen oxides of A & B synthesis: A + B C decomposition: AB A + B single displacement: A + BC AC + B double displacement: AB + CD AD + CB

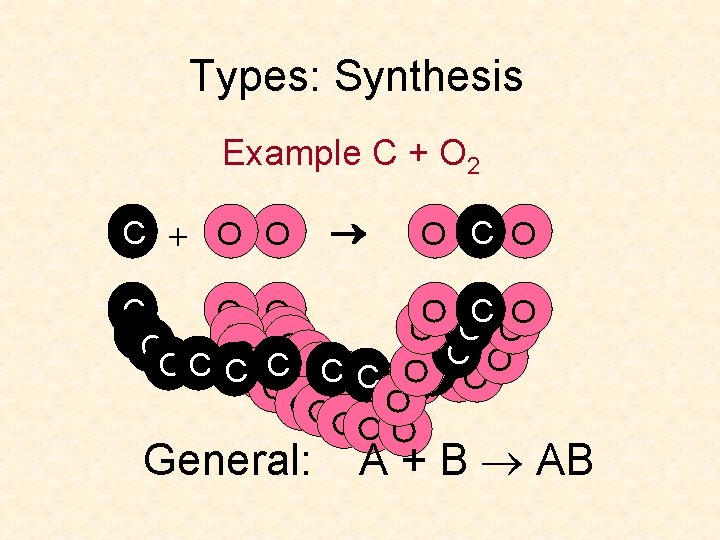
Types: Synthesis Example C + O 2 C + O O O C O CC O O O C C C OC OOC C OCO OO CO O O OO OO OOO General: A + B AB

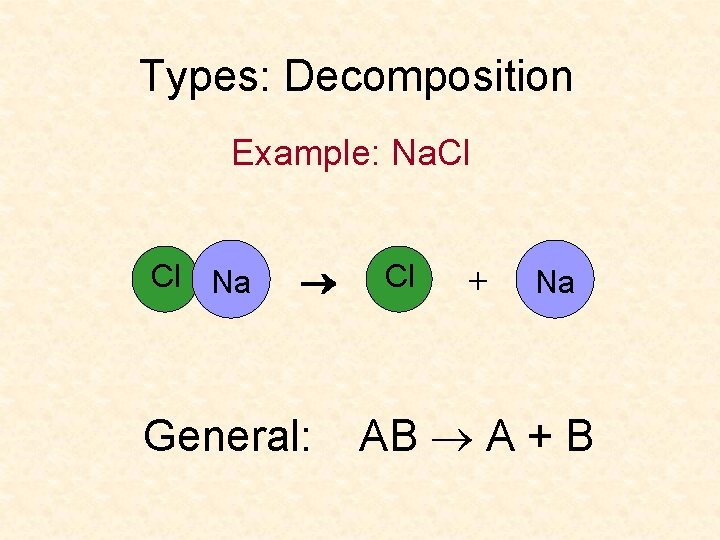
Types: Decomposition Example: Na. Cl Cl Na General: Cl + Na AB A + B

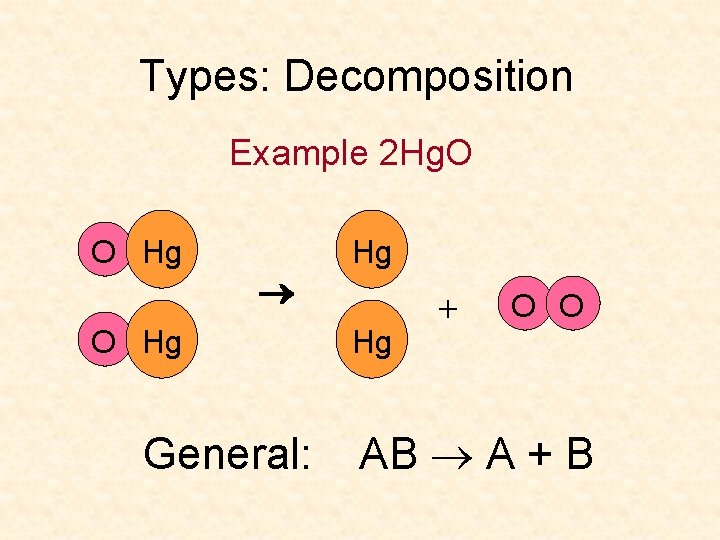
Types: Decomposition Example 2 Hg. O O Hg General: Hg Hg + O O AB A + B

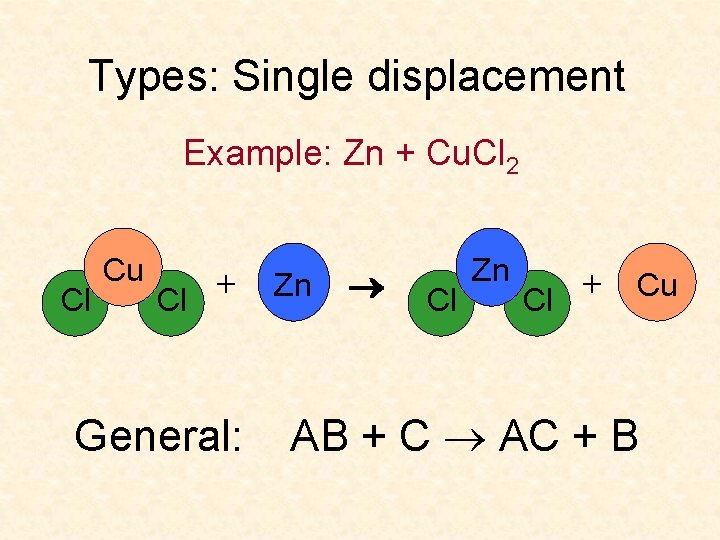
Types: Single displacement Example: Zn + Cu. Cl 2 Cl Cu + Cl General: Zn Cl Zn + Cu Cl AB + C AC + B

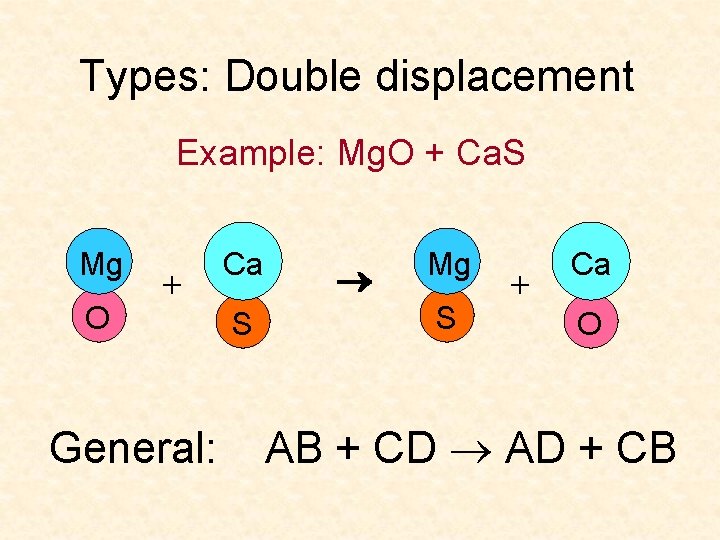
Types: Double displacement Example: Mg. O + Ca. S Mg O + General: Ca S Mg S + Ca O AB + CD AD + CB

Chemical Reactions Involving Oxygen 3. combustion: AB + oxygen oxides of A & B synthesis: A + B C decomposition: AB A + B single displacement: A + BC AC + B double displacement: AB + CD AD + CB 4. Part 1 reactions can be described as both combustion and synthesis 5. Litmus turned red. Non-metal oxides react with water to form acids. 6. Oxygen (O 2). A chemical change. If it were a physical change of state it would keep bubbling until it all evaporated (not turn solid)

Chemical Reactions Involving Oxygen 7. This is a decomposition reaction potassium chlorate potassium chloride + oxygen 8. a) Single displacement b) Decomposition c) Synthesis d) Double displacement e) Single displacement f) Double displacement g) Combustion (and double displacement? ) For more lessons, visit www. chalkbored. com