Chemical Reactions in Cells To keep your body
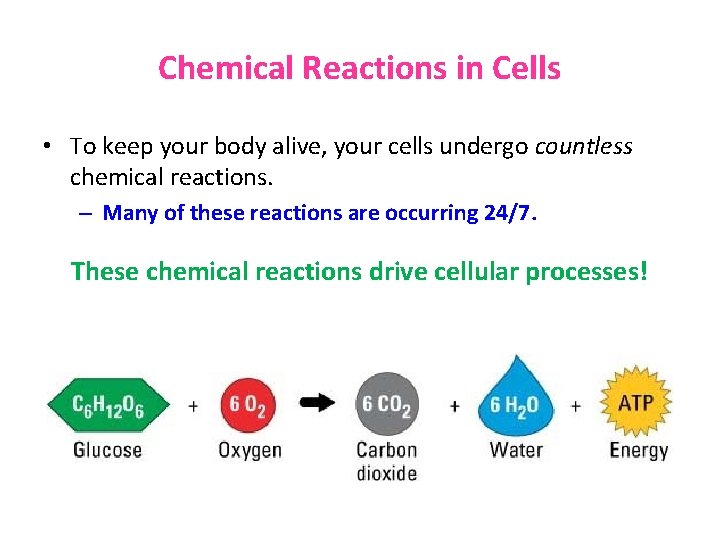
Chemical Reactions in Cells • To keep your body alive, your cells undergo countless chemical reactions. – Many of these reactions are occurring 24/7. These chemical reactions drive cellular processes!

Cells in your body produce CO 2, then blood carries the CO 2 from the cells to your lungs (you exhale it out). PROBLEM • CO 2 is not soluble (dissolvable) in water, so it cannot be carried through your blood. A chemical reaction in your body converts CO 2 into a soluble compound.
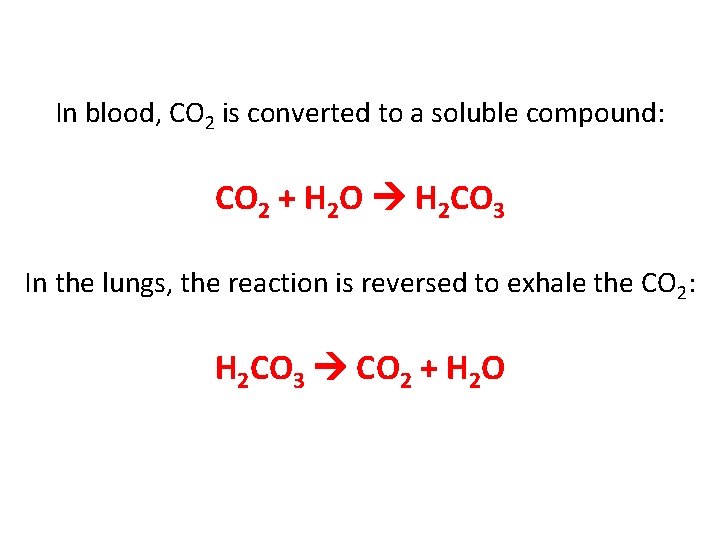
In blood, CO 2 is converted to a soluble compound: CO 2 + H 2 O H 2 CO 3 In the lungs, the reaction is reversed to exhale the CO 2: H 2 CO 3 CO 2 + H 2 O
Chemical Reactions & Enzymes
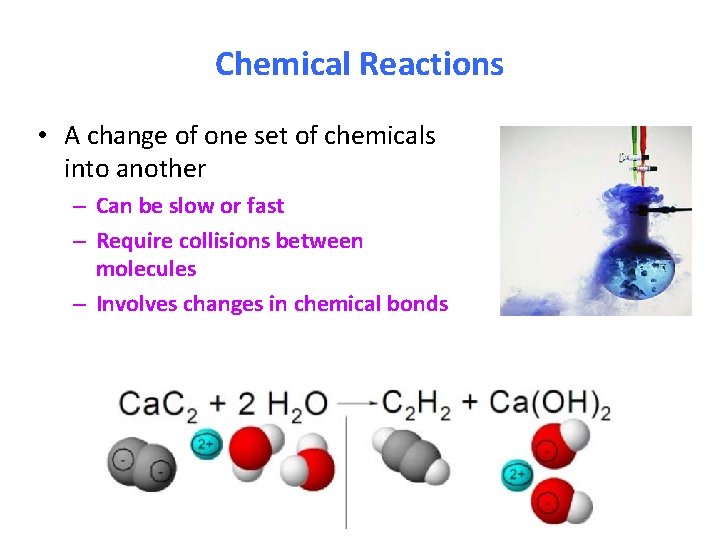
Chemical Reactions • A change of one set of chemicals into another – Can be slow or fast – Require collisions between molecules – Involves changes in chemical bonds
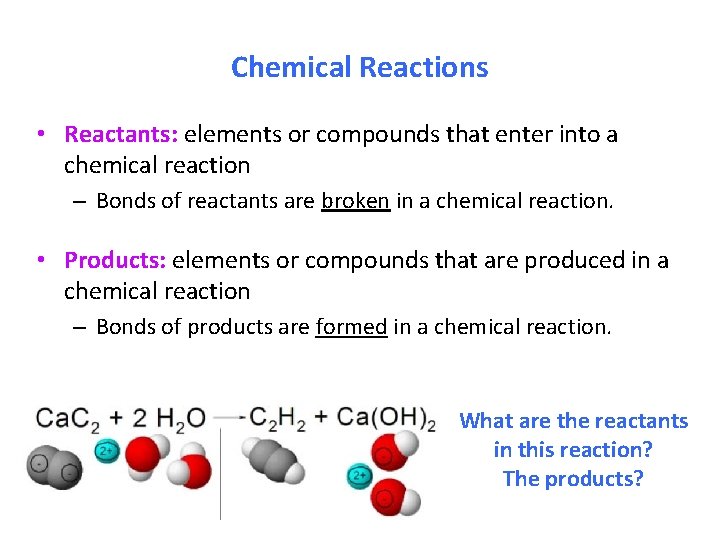
Chemical Reactions • Reactants: elements or compounds that enter into a chemical reaction – Bonds of reactants are broken in a chemical reaction. • Products: elements or compounds that are produced in a chemical reaction – Bonds of products are formed in a chemical reaction. What are the reactants in this reaction? The products?
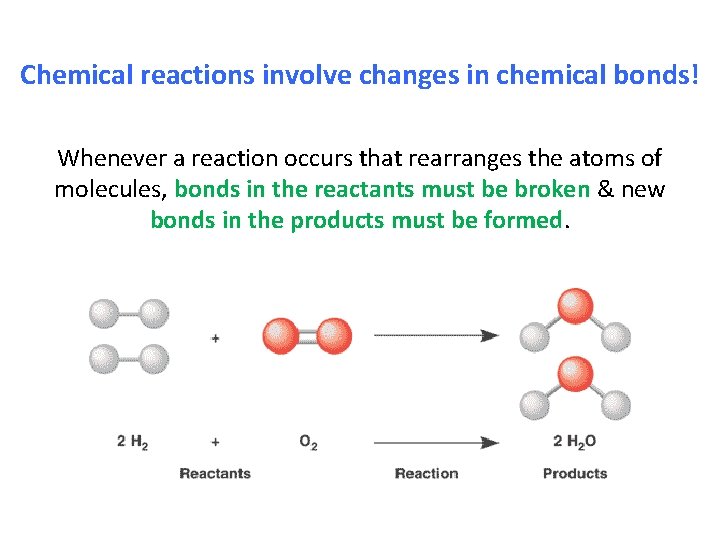
Chemical reactions involve changes in chemical bonds! Whenever a reaction occurs that rearranges the atoms of molecules, bonds in the reactants must be broken & new bonds in the products must be formed.

Chemical Reactions & Energy • Breaking & forming chemical bonds requires energy release or absorption. • Reactions that release energy can occur spontaneously (but not all do). – Energy is released as heat. • Reactions that absorb energy will not occur without an energy source.
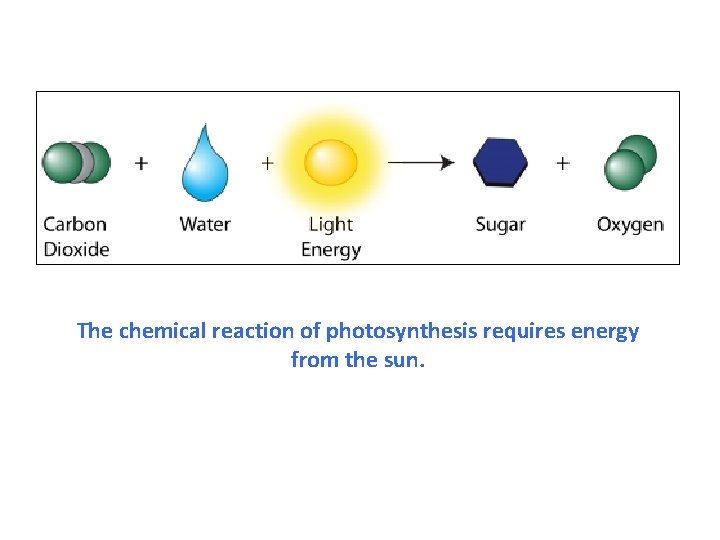
The chemical reaction of photosynthesis requires energy from the sun.
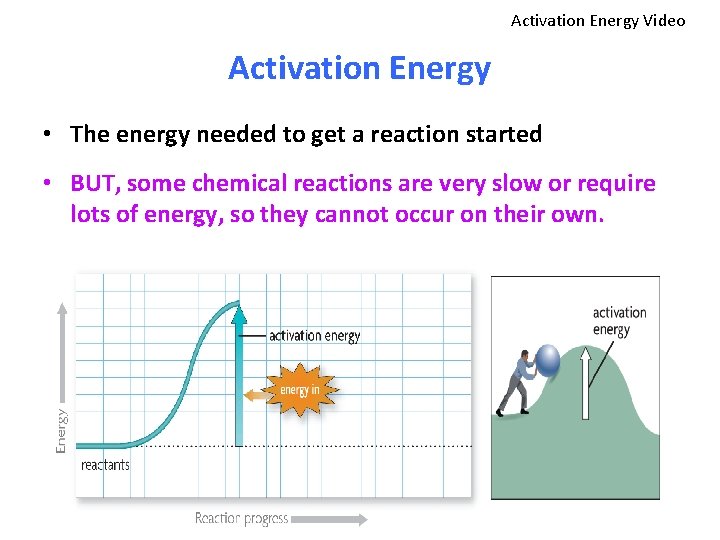
Activation Energy Video Activation Energy • The energy needed to get a reaction started • BUT, some chemical reactions are very slow or require lots of energy, so they cannot occur on their own.
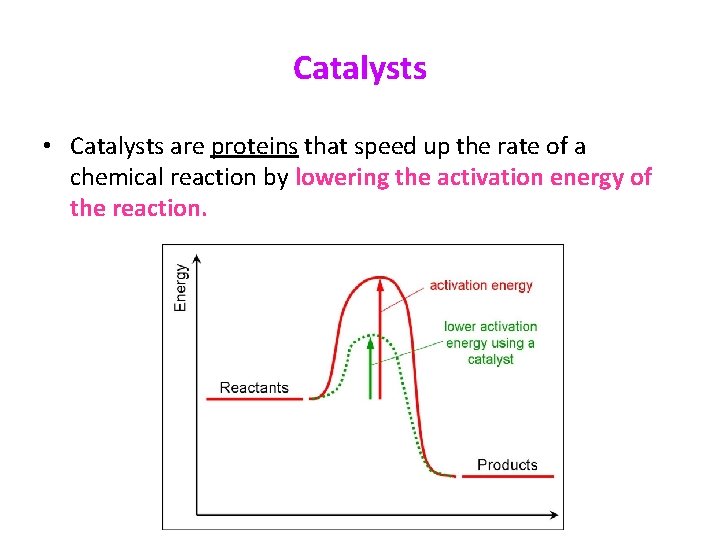
Catalysts • Catalysts are proteins that speed up the rate of a chemical reaction by lowering the activation energy of the reaction.
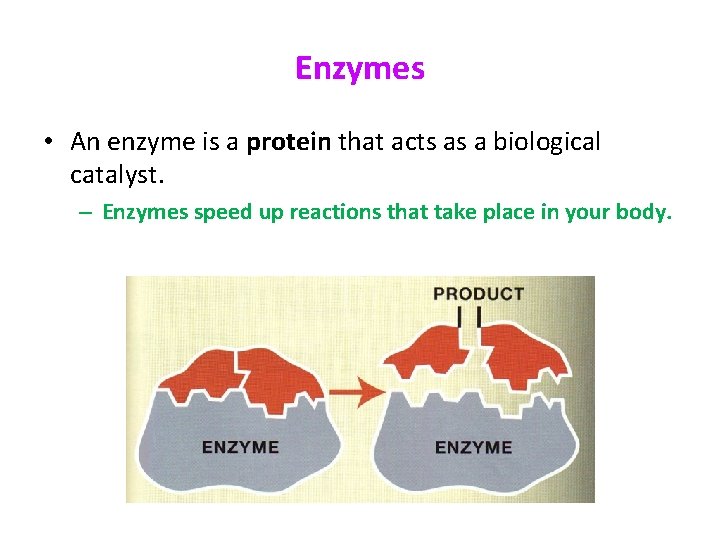
Enzymes • An enzyme is a protein that acts as a biological catalyst. – Enzymes speed up reactions that take place in your body.
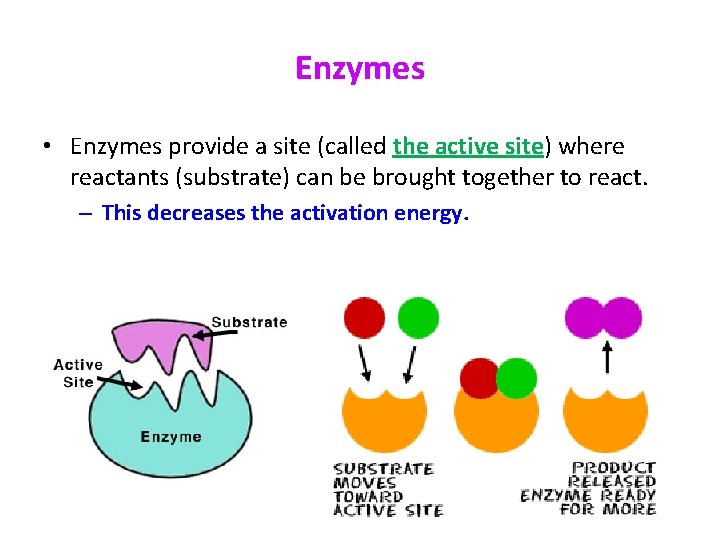
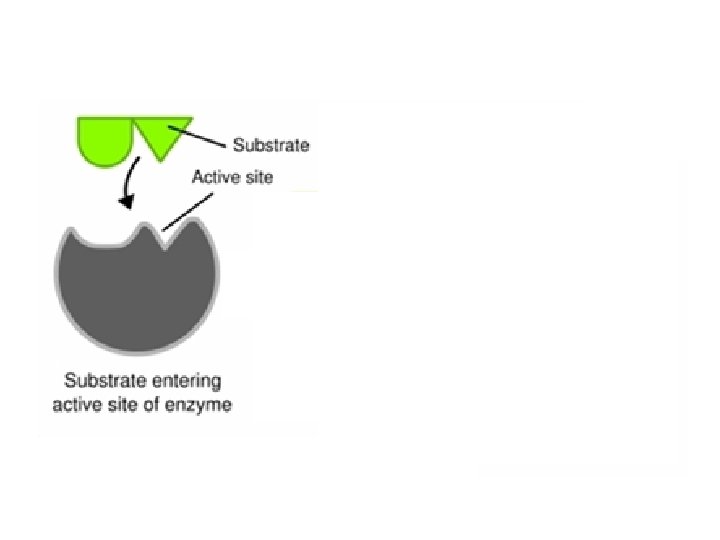
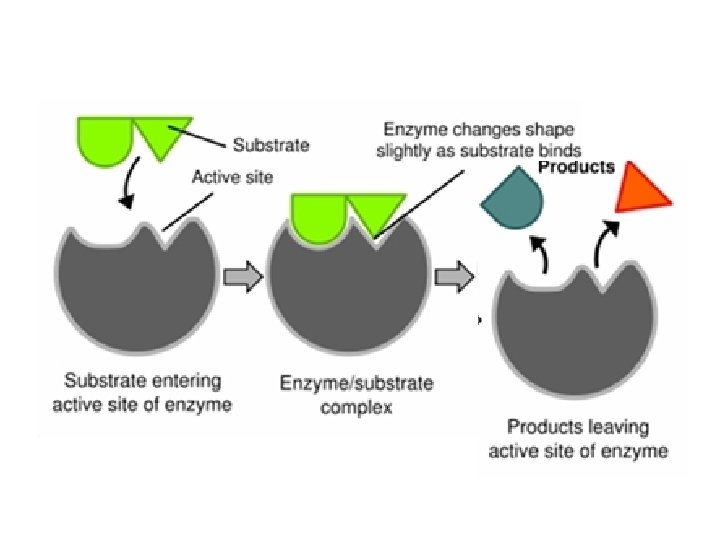
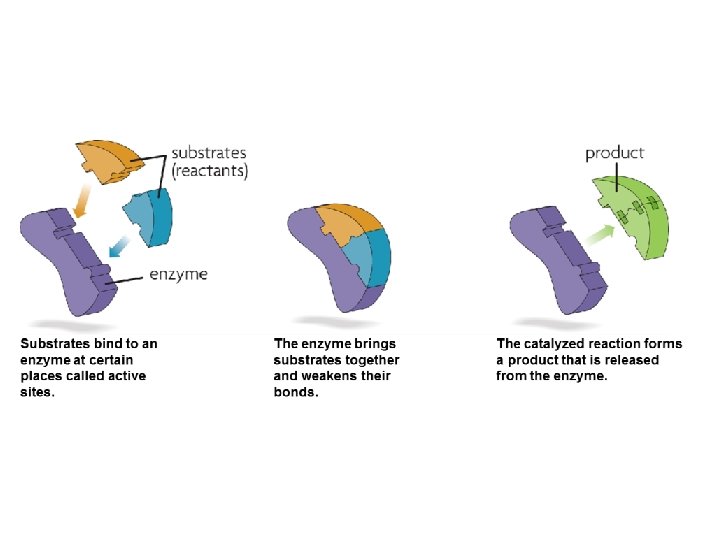
Enzymes • Enzymes provide a site (called the active site) where reactants (substrate) can be brought together to react. – This decreases the activation energy.
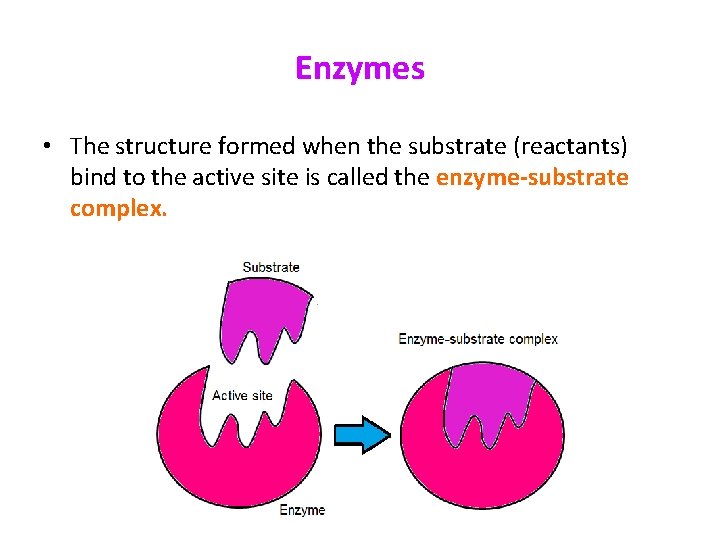
Enzymes • The structure formed when the substrate (reactants) bind to the active site is called the enzyme-substrate complex.
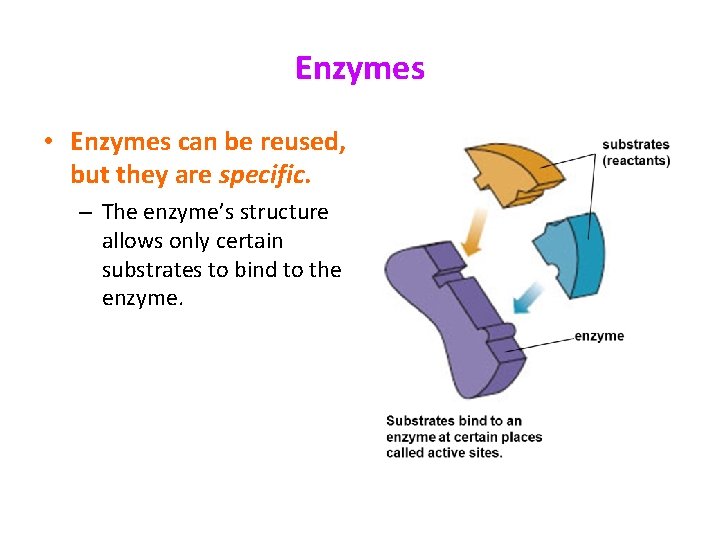
Enzymes • Enzymes can be reused, but they are specific. – The enzyme’s structure allows only certain substrates to bind to the enzyme.
The enzyme-substrate relationship is like a lock & key! Only the correctly shaped key will open the lock…
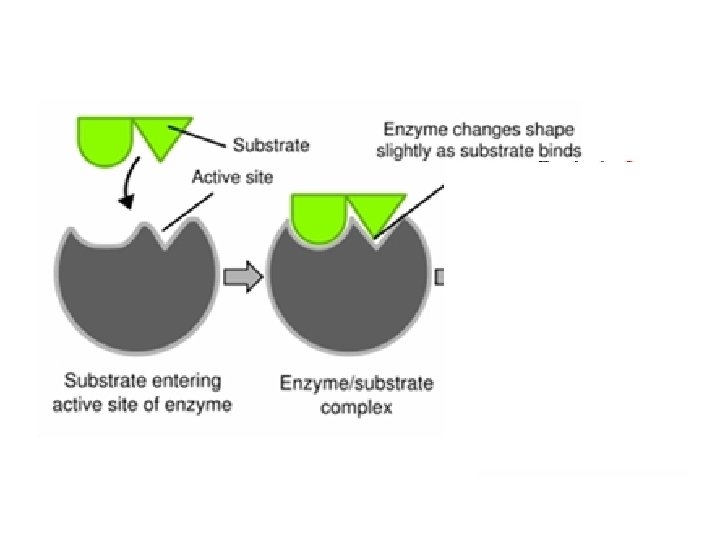
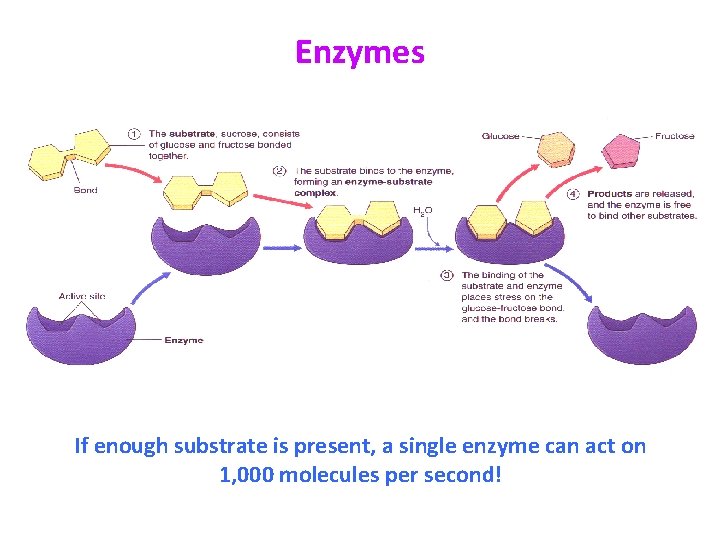
Enzymes If enough substrate is present, a single enzyme can act on 1, 000 molecules per second!
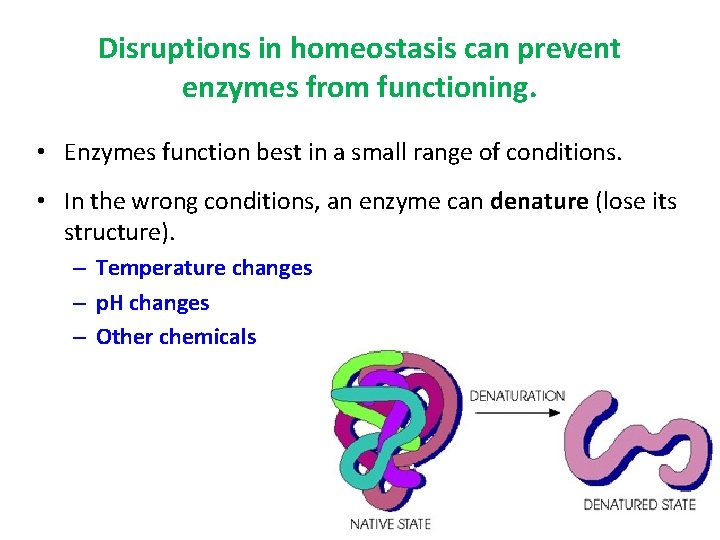
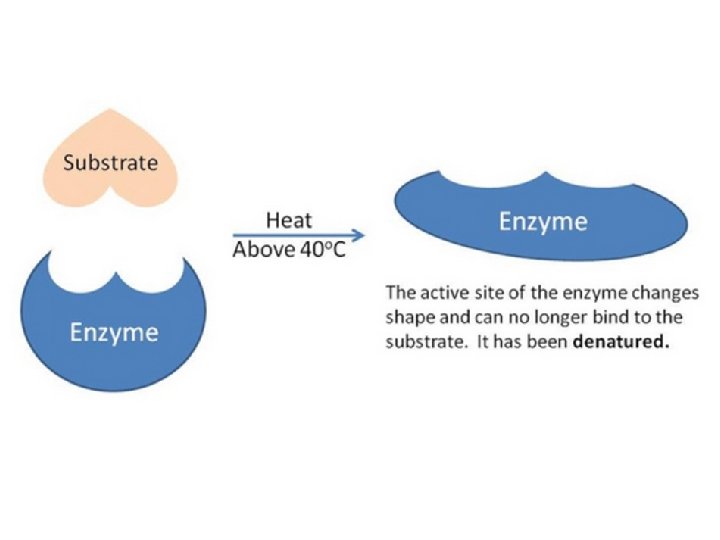
Disruptions in homeostasis can prevent enzymes from functioning. • Enzymes function best in a small range of conditions. • In the wrong conditions, an enzyme can denature (lose its structure). – Temperature changes – p. H changes – Other chemicals
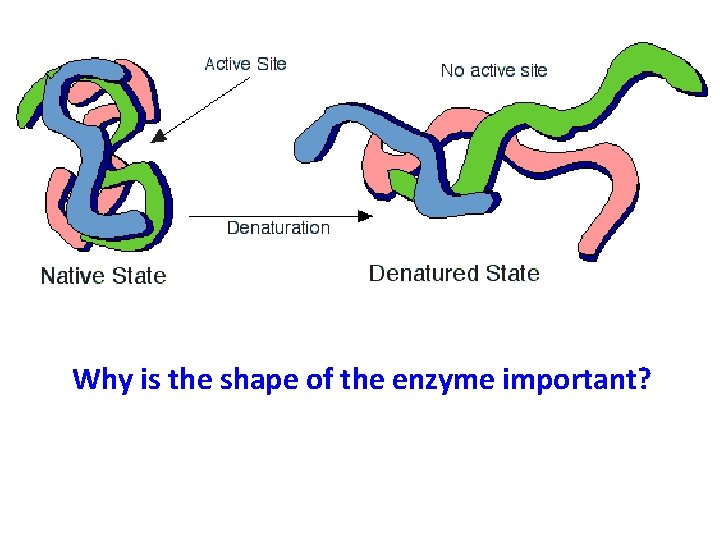
Why is the shape of the enzyme important?
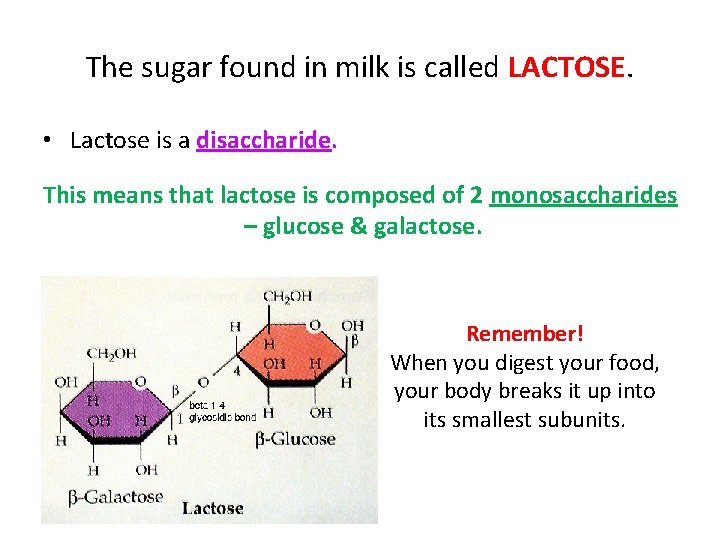
The sugar found in milk is called LACTOSE. • Lactose is a disaccharide. This means that lactose is composed of 2 monosaccharides – glucose & galactose. Remember! When you digest your food, your body breaks it up into its smallest subunits.

Do we need the enzyme LACTASE to digest milk?
- Slides: 26