Chemical Reactions Formation of Solid Also called precipitate


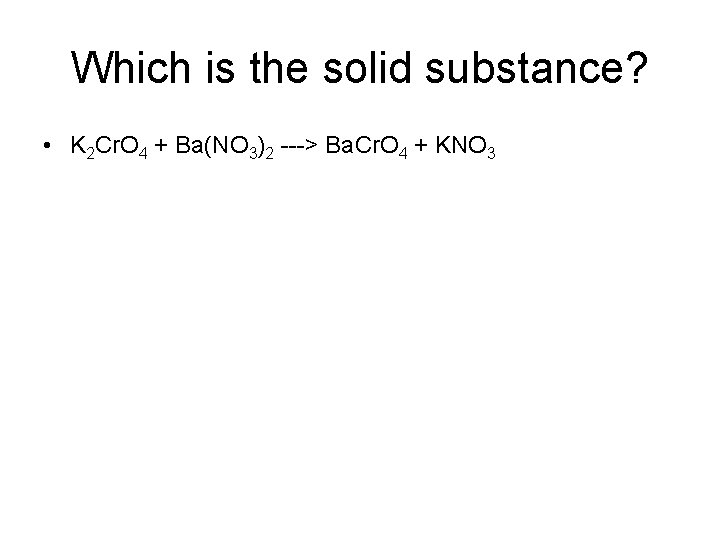

- Slides: 16

Chemical Reactions

Formation of Solid • Also called precipitate K 2 Cr. O 4(aq)+ Ba(NO 3)2(aq)-->forms a yellow solid substance • How to identify the solid that forms in a precipitation reaction? know what happens when an ionic compounds dissolve in water Ba(NO 3)2 ---- > Ba 2+ NO 3 K 2 Cr. O 4 ----> K+ Cr. O 42 - substances that dissolves in water and produces separated ions are called strong electrolytes 3/3/2021 2

K 2 Cr. O 4 Ba(NO 3)2 • 2 K+(aq) + Cr. O 42 -(aq) + Ba 2+ + 2 NO 3 How to decide what products form? Recall that a solid compound must have a zero net charge. The products must contain both anion and cation 3/3/2021 3

Example K 2 Cr. O 4 Ba(NO 3)2 2 K+(aq) + Cr. O 42 -(aq) + Ba 2+ + 2 NO 3 K+ and Ba+ could not combine Cr. O 42 - and NO 3 - could not combine It should be a combination of positive and negative. Therefore; K 2 Cr. O 4 + Ba(NO 3)2 ---> Ba. Cr. O 4 + 2 KNO 3 3/3/2021 4

Using Solubility Rules • Soluble – dissolves in water • Insoluble / slightly soluble dissolves tiny amounts that is undetectable to the naked eye 3/3/2021 5

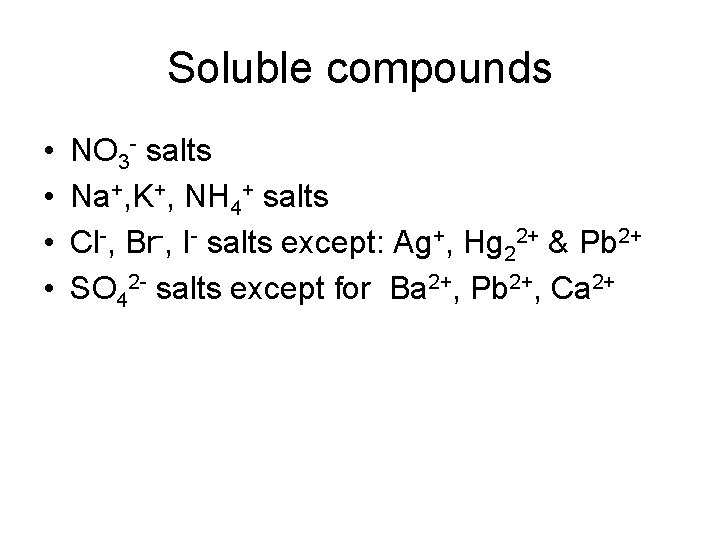
Soluble compounds • • NO 3 - salts Na+, K+, NH 4+ salts Cl-, Br-, I- salts except: Ag+, Hg 22+ & Pb 2+ SO 42 - salts except for Ba 2+, Pb 2+, Ca 2+
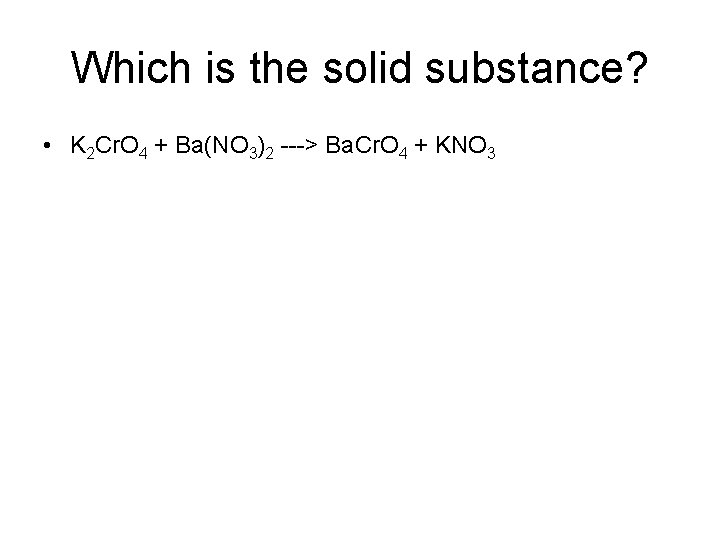
Which is the solid substance? • K 2 Cr. O 4 + Ba(NO 3)2 ---> Ba. Cr. O 4 + KNO 3

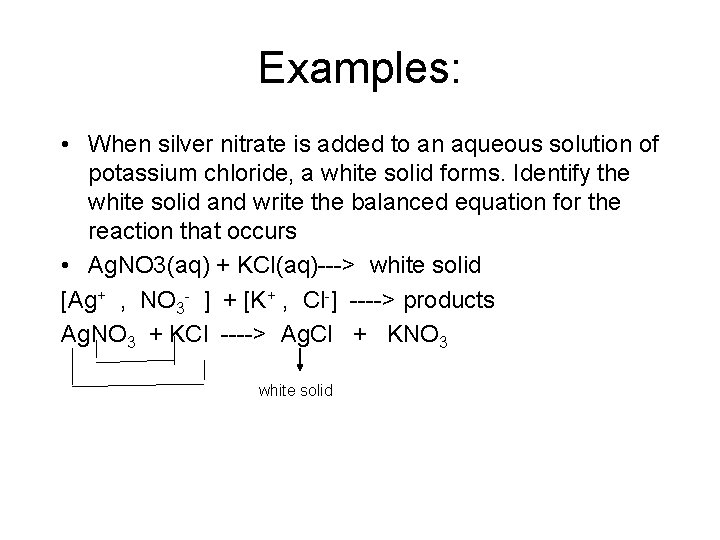
Examples: • When silver nitrate is added to an aqueous solution of potassium chloride, a white solid forms. Identify the white solid and write the balanced equation for the reaction that occurs • Ag. NO 3(aq) + KCl(aq)---> white solid [Ag+ , NO 3 - ] + [K+ , Cl-] ----> products Ag. NO 3 + KCl ----> Ag. Cl + KNO 3 white solid

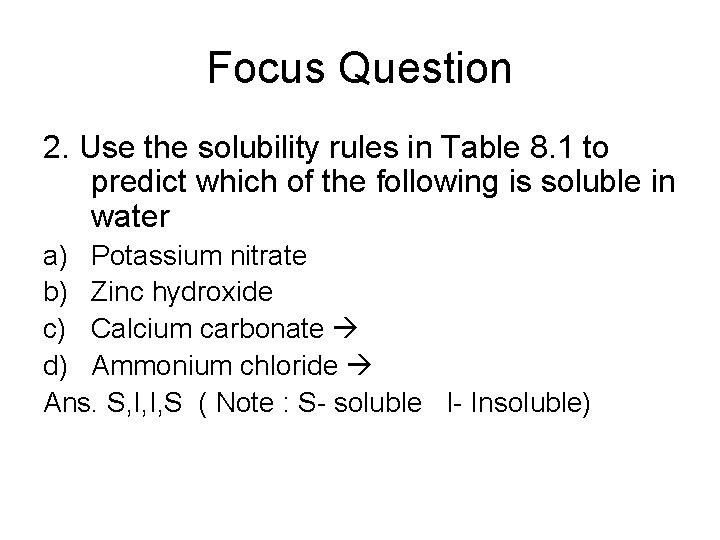
Focus Question 2. Use the solubility rules in Table 8. 1 to predict which of the following is soluble in water a) Potassium nitrate b) Zinc hydroxide c) Calcium carbonate d) Ammonium chloride Ans. S, I, I, S ( Note : S- soluble I- Insoluble)

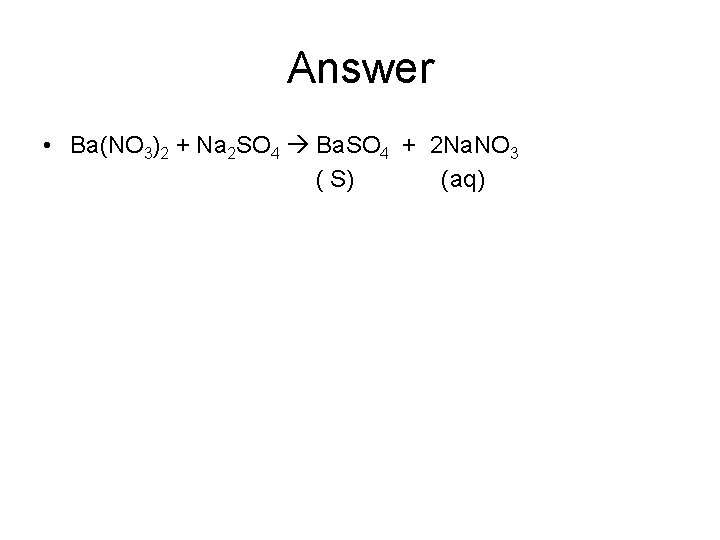
Practice problems • When an aqueous solution of barium nitrate is added to an aqueous solution of sodium sulfate, a white solid forms. Identify the white solid and write the balanced equation for the reaction that occurs

Answer • Ba(NO 3)2 + Na 2 SO 4 Ba. SO 4 + 2 Na. NO 3 ( S) (aq)

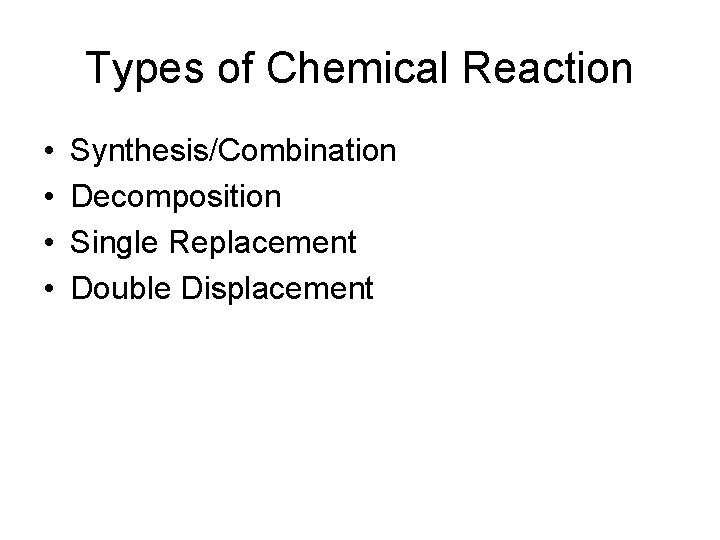
Types of Chemical Reaction • • Synthesis/Combination Decomposition Single Replacement Double Displacement

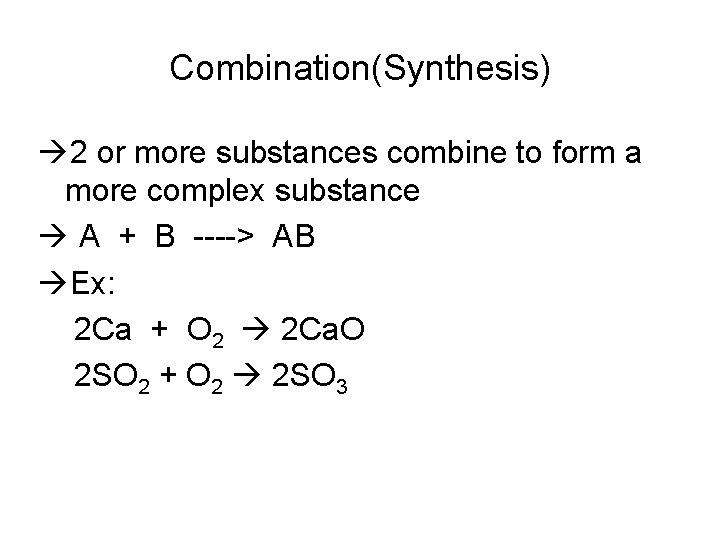
Combination(Synthesis) 2 or more substances combine to form a more complex substance A + B ----> AB Ex: 2 Ca + O 2 2 Ca. O 2 SO 2 + O 2 2 SO 3

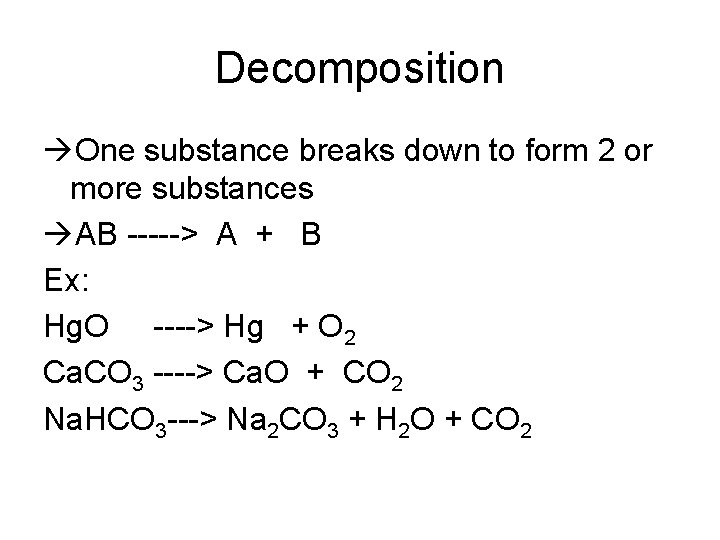
Decomposition One substance breaks down to form 2 or more substances AB -----> A + B Ex: Hg. O ----> Hg + O 2 Ca. CO 3 ----> Ca. O + CO 2 Na. HCO 3 ---> Na 2 CO 3 + H 2 O + CO 2

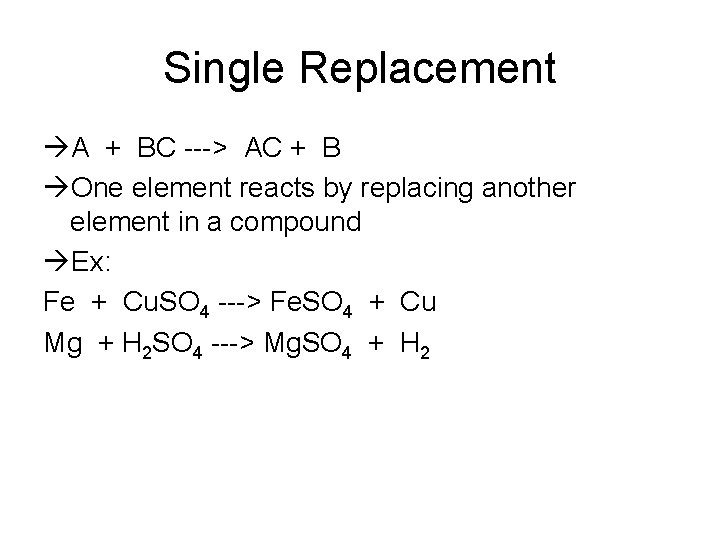
Single Replacement A + BC ---> AC + B One element reacts by replacing another element in a compound Ex: Fe + Cu. SO 4 ---> Fe. SO 4 + Cu Mg + H 2 SO 4 ---> Mg. SO 4 + H 2

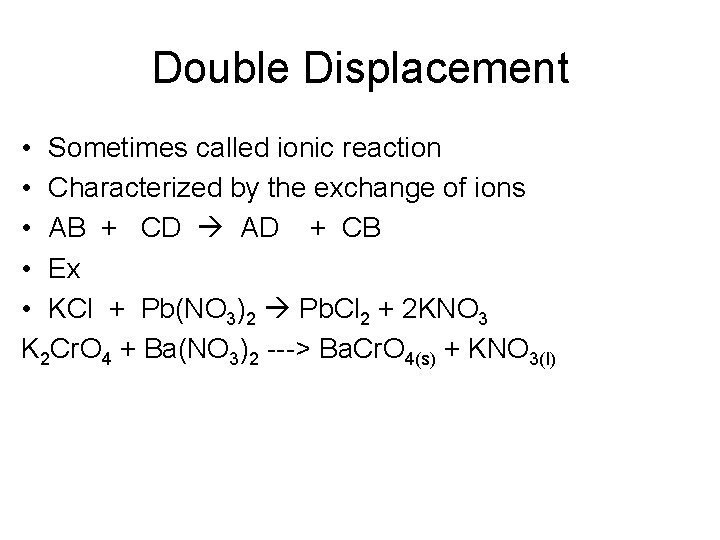
Double Displacement • Sometimes called ionic reaction • Characterized by the exchange of ions • AB + CD AD + CB • Ex • KCl + Pb(NO 3)2 Pb. Cl 2 + 2 KNO 3 K 2 Cr. O 4 + Ba(NO 3)2 ---> Ba. Cr. O 4(s) + KNO 3(l)