Chemical Reactions Evidence of Chemical Reactions n n



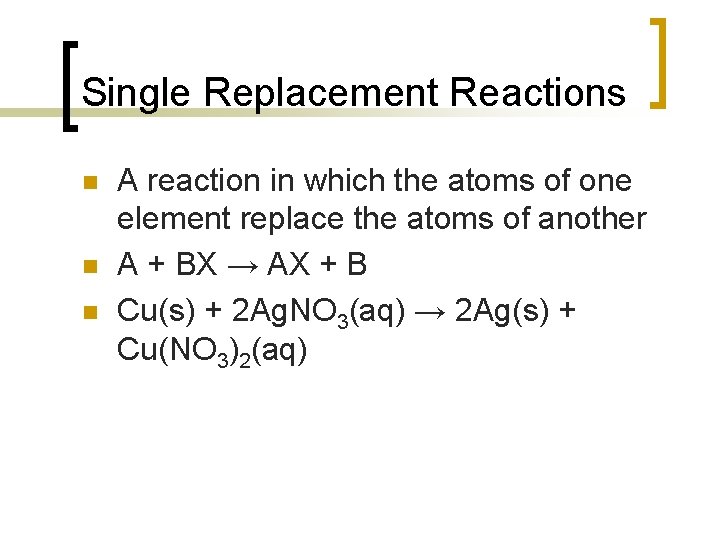
- Slides: 16

Chemical Reactions

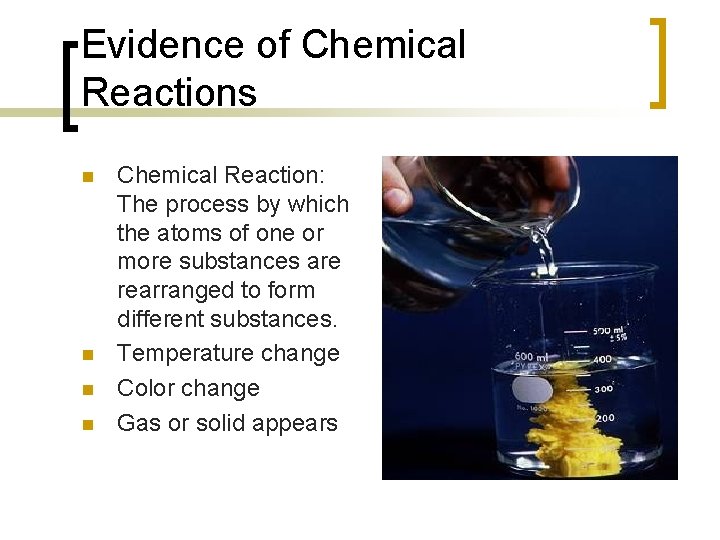
Evidence of Chemical Reactions n n Chemical Reaction: The process by which the atoms of one or more substances are rearranged to form different substances. Temperature change Color change Gas or solid appears

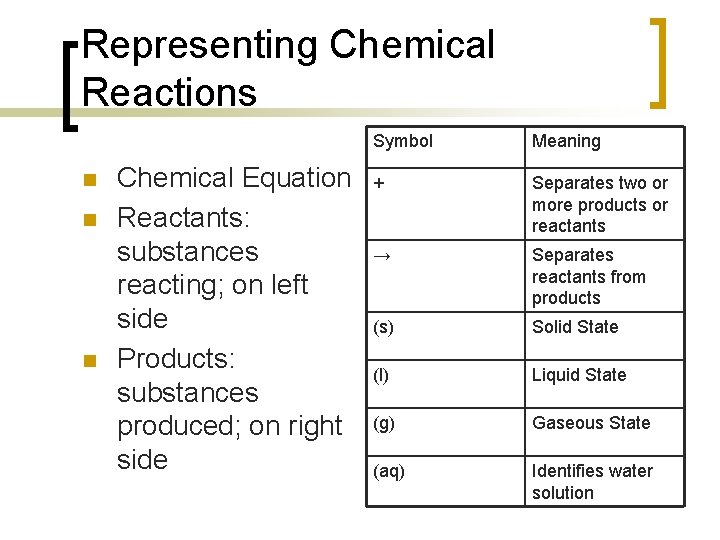
Representing Chemical Reactions n n n Chemical Equation Reactants: substances reacting; on left side Products: substances produced; on right side Symbol Meaning + Separates two or more products or reactants → Separates reactants from products (s) Solid State (l) Liquid State (g) Gaseous State (aq) Identifies water solution

Representing Chemical Reactions n n How would you write an equation for sodium reacting with chloride to make sodium chloride? Reactant 1 + Reactant 2 → Product 1 sodium + chloride → sodium chloride Na (s) + Cl 2 (g) → Na. Cl (s)

Representing Chemical Reactions n n n Law of Conservation of Mass: atoms cannot be created or destroyed. All atoms in reactants must be in products, and visa versa Balancing the equation Na (s) + Cl 2 (g) → Na. Cl (s) Can not occur without coefficients: number written in front

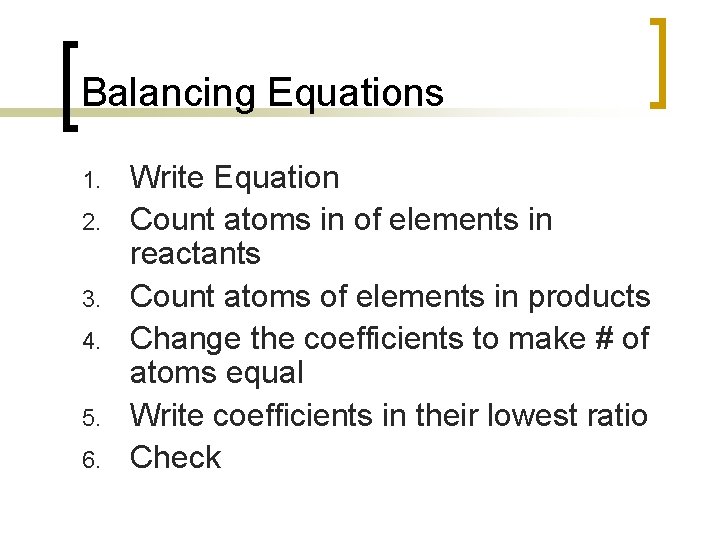
Balancing Equations 1. 2. 3. 4. 5. 6. Write Equation Count atoms in of elements in reactants Count atoms of elements in products Change the coefficients to make # of atoms equal Write coefficients in their lowest ratio Check

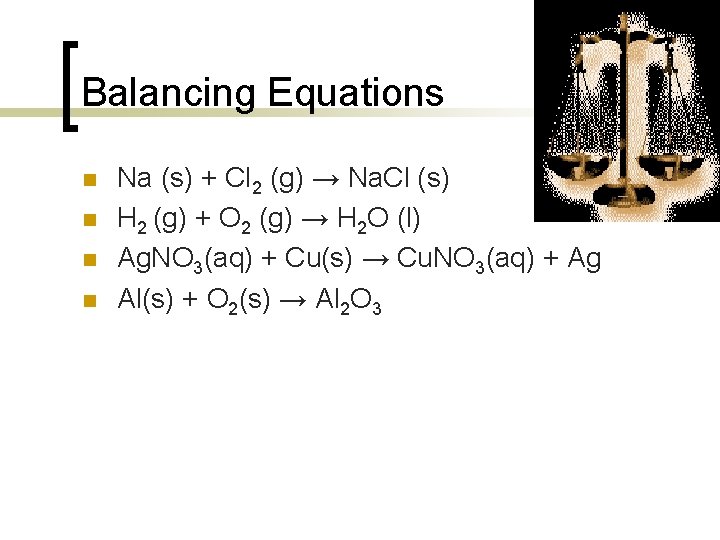
Balancing Equations n n Na (s) + Cl 2 (g) → Na. Cl (s) H 2 (g) + O 2 (g) → H 2 O (l) Ag. NO 3(aq) + Cu(s) → Cu. NO 3(aq) + Ag Al(s) + O 2(s) → Al 2 O 3

Classifying Chemical Reactions n n n 5 types Need to be able to recognize different types. Need to be able to predict products of different types of reactions

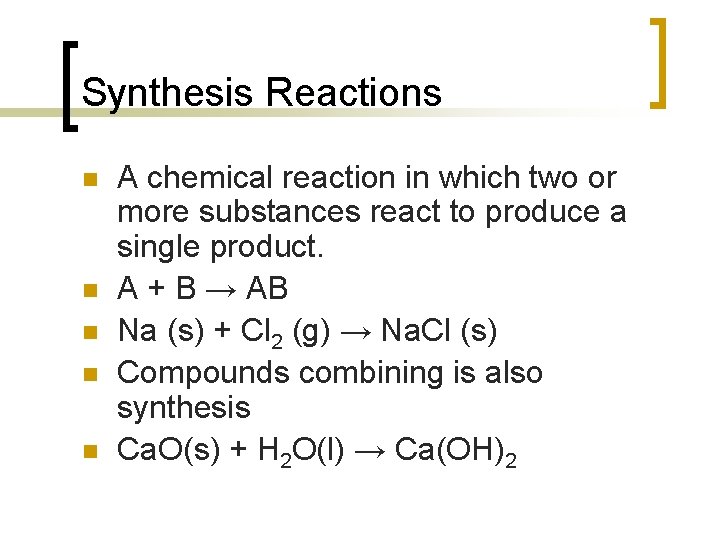
Synthesis Reactions n n n A chemical reaction in which two or more substances react to produce a single product. A + B → AB Na (s) + Cl 2 (g) → Na. Cl (s) Compounds combining is also synthesis Ca. O(s) + H 2 O(l) → Ca(OH)2

Combustion Reaction n Oxygen combines with a substance to produce heat and energy 2 H 2(g) + O 2(g) → 2 H 2 O(g) All combustion are synthesis not all synthesis are combustion.

Combustion Reaction n n CH 4, methane, is a hydrocarbon + oxygen → carbon dioxide + water CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) Can be tough to balance, always balance O 2 last.

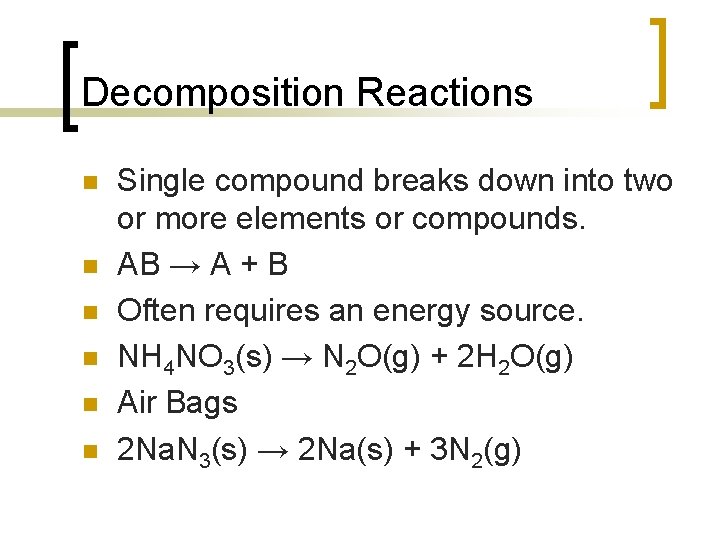
Decomposition Reactions n n n Single compound breaks down into two or more elements or compounds. AB → A + B Often requires an energy source. NH 4 NO 3(s) → N 2 O(g) + 2 H 2 O(g) Air Bags 2 Na. N 3(s) → 2 Na(s) + 3 N 2(g)
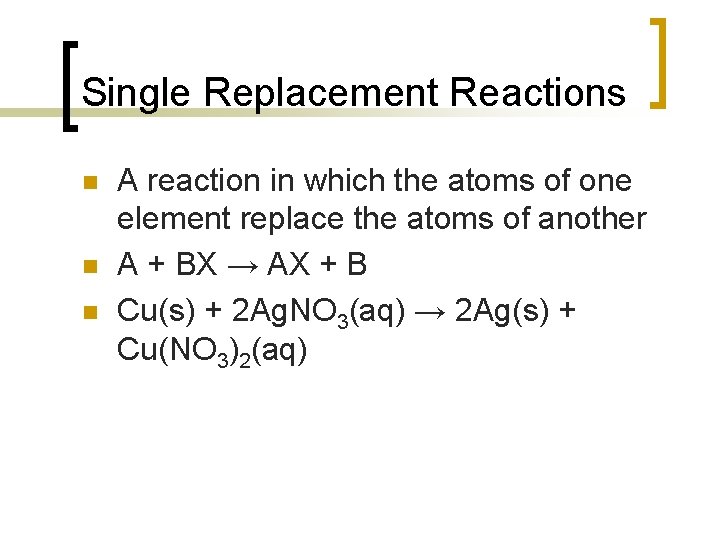
Single Replacement Reactions n n n A reaction in which the atoms of one element replace the atoms of another A + BX → AX + B Cu(s) + 2 Ag. NO 3(aq) → 2 Ag(s) + Cu(NO 3)2(aq)

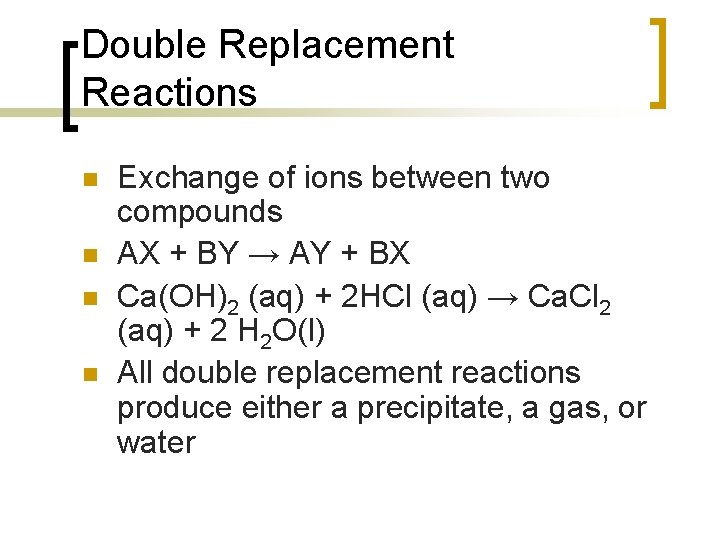
Double Replacement Reactions n n Exchange of ions between two compounds AX + BY → AY + BX Ca(OH)2 (aq) + 2 HCl (aq) → Ca. Cl 2 (aq) + 2 H 2 O(l) All double replacement reactions produce either a precipitate, a gas, or water

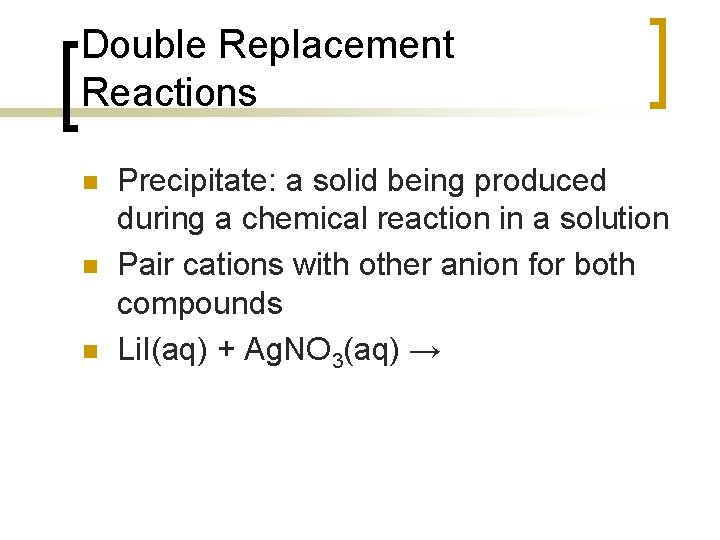
Double Replacement Reactions n n n Precipitate: a solid being produced during a chemical reaction in a solution Pair cations with other anion for both compounds Li. I(aq) + Ag. NO 3(aq) →

Types of reactions