CHEMICAL REACTIONS Equations Types of Reactions Prediction of










- Slides: 26

CHEMICAL REACTIONS • Equations • Types of Reactions • Prediction of Products • Diatomic Molecules • Solubility Rules

Chemical Reactions • Substances undergo changes to produce new substances with different properties • Represented by chemical equations • Obey the law of conservation of mass • Can be endothermic or exothermic

Evidence of a Chemical Reaction • Change in temperature • Formation of a precipitate (insoluble compound) • Change in color • Smoke • Bubbles • Production of light

Chemical Equations • Use symbols and formulas to represent chemical reactions • Include state symbols: – (s) solids – (l) liquids – (g) gases – (aq) solid dissolved in water…see solubility chart

Special Elements • Diatomic Molecules – – – – Hydrogen Nitrogen Oxygen Fluorine Chlorine Bromine Iodine • Others – Phosphorus (P 4) – Sulfur (S 8)

Parts of a Chemical Equation • Reactants – Substances that enter into a reaction • Products – Substances that are produced by a reaction • State symbols – Indicate the state of matter for each substance in the reaction • Coefficients – Indicate the number of molecules or atoms of each substance in a reaction

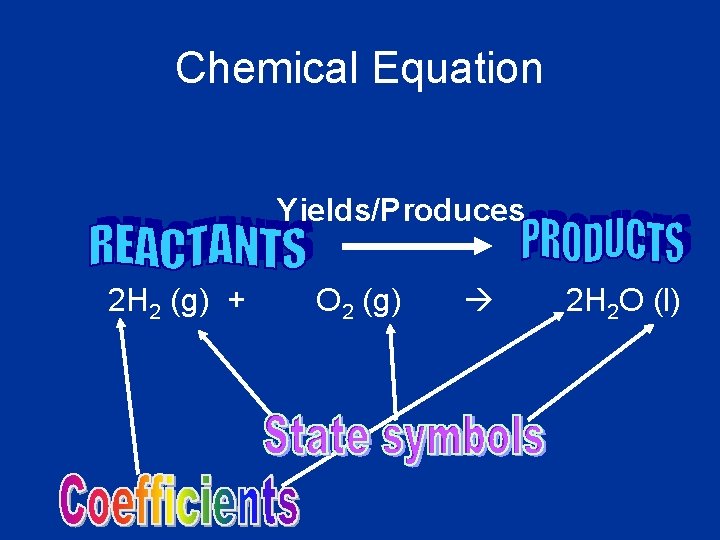
Chemical Equation Yields/Produces 2 H 2 (g) + O 2 (g) 2 H 2 O (l)

Why Balance? • Equations must be balanced in order to satisfy the law of conservation of mass • According to “the law” the mass of the reactants must be equal to the mass of the products

Find Masses 2 H 2 (g) + O 2 (g) 2 H 2 O (l)

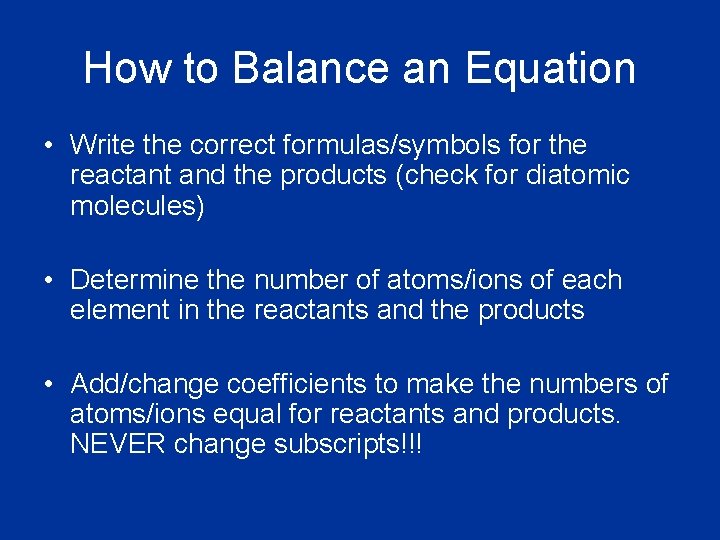
How to Balance an Equation • Write the correct formulas/symbols for the reactant and the products (check for diatomic molecules) • Determine the number of atoms/ions of each element in the reactants and the products • Add/change coefficients to make the numbers of atoms/ions equal for reactants and products. NEVER change subscripts!!!

Helpful Hints • Balance oxygen last and hydrogen next to last • Keep polyatomic ions “together” as long as they are together in the reactants and in the products

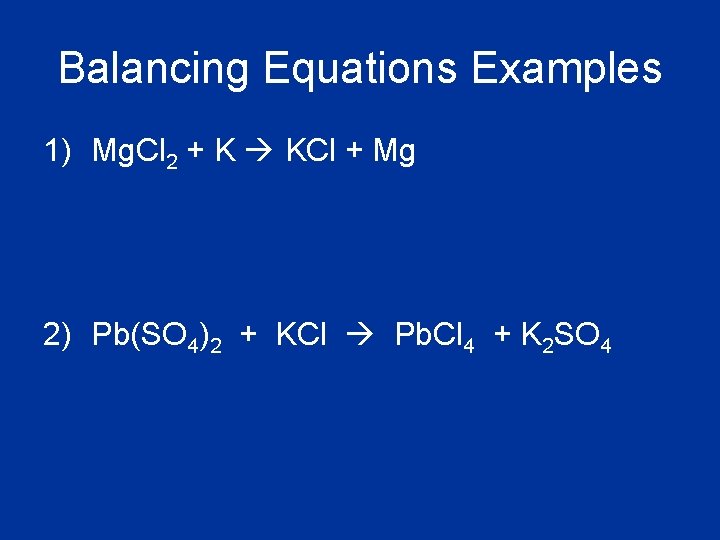
Balancing Equations Examples 1) Mg. Cl 2 + K KCl + Mg 2) Pb(SO 4)2 + KCl Pb. Cl 4 + K 2 SO 4

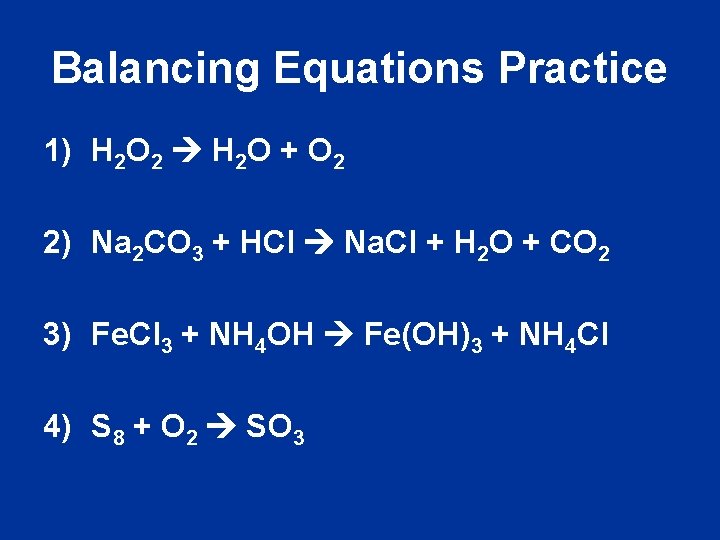
Balancing Equations Practice 1) H 2 O 2 H 2 O + O 2 2) Na 2 CO 3 + HCl Na. Cl + H 2 O + CO 2 3) Fe. Cl 3 + NH 4 OH Fe(OH)3 + NH 4 Cl 4) S 8 + O 2 SO 3

Word Equations • Expresses a chemical reaction in words rather than using formulas and symbols • You will need to be able to convert from word equations to chemical equations and vice versa

Write word equations for: 1) H 2 O 2 H 2 O + O 2 2) Na 2 CO 3 + HCl Na. Cl + H 2 O + CO 2 3) Fe. Cl 3 + NH 4 OH Fe(OH)3 + NH 4 Cl 4) S 8 + O 2 SO 3

Types of Reactions • Synthesis • Decomposition • Single-Replacement • Double-Replacement • Combustion

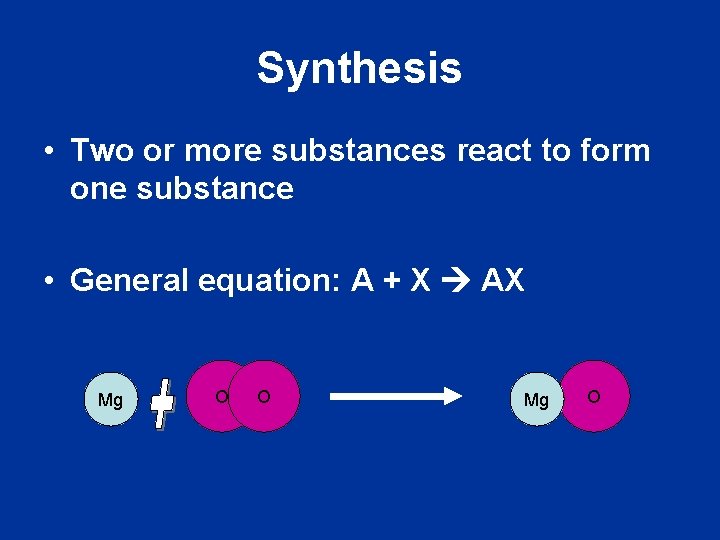
Synthesis • Two or more substances react to form one substance • General equation: A + X AX Mg O O Mg O

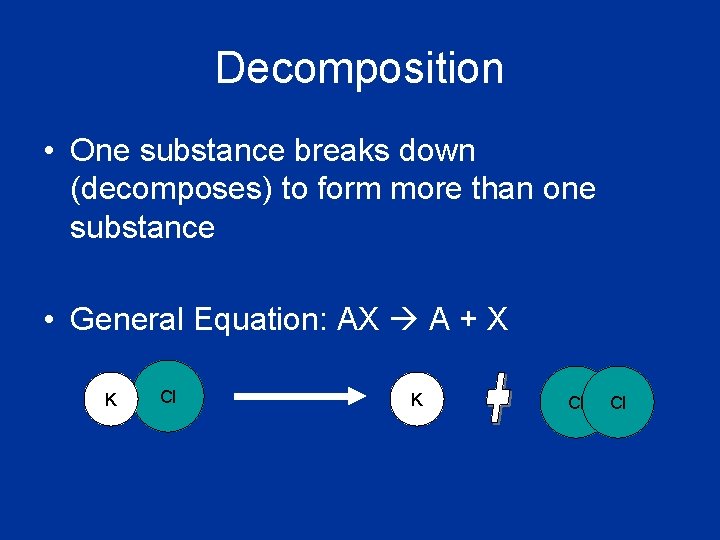
Decomposition • One substance breaks down (decomposes) to form more than one substance • General Equation: AX A + X K Cl Cl

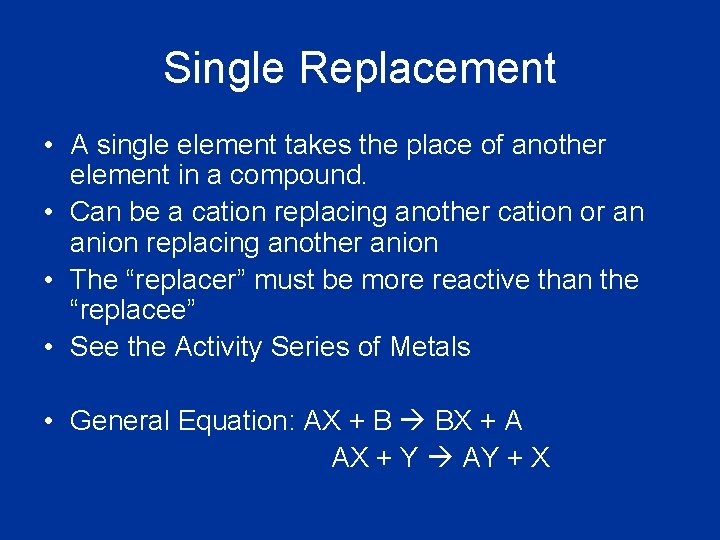
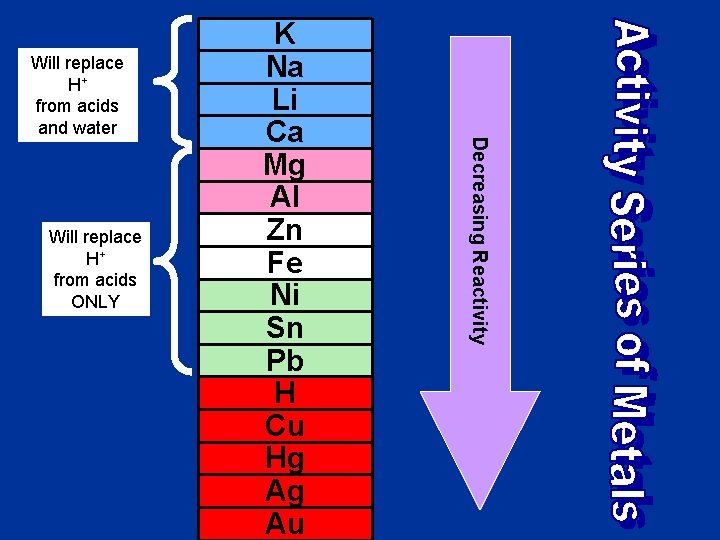
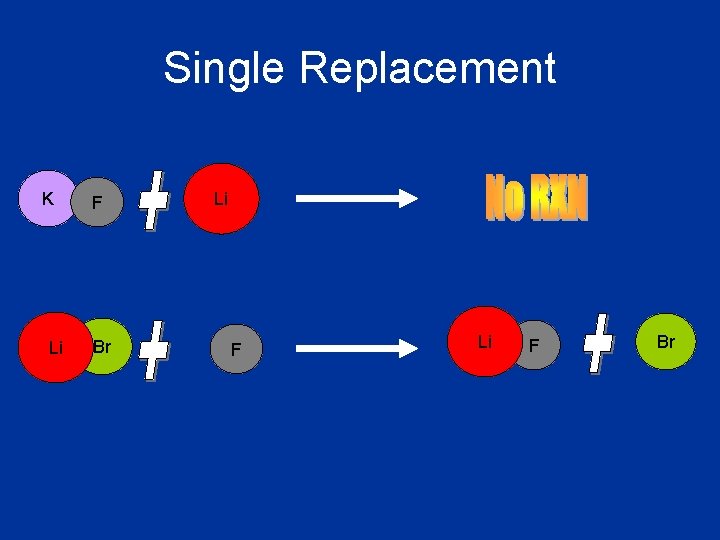
Single Replacement • A single element takes the place of another element in a compound. • Can be a cation replacing another cation or an anion replacing another anion • The “replacer” must be more reactive than the “replacee” • See the Activity Series of Metals • General Equation: AX + B BX + A AX + Y AY + X

Will replace H+ from acids ONLY Decreasing Reactivity Will replace H+ from acids and water K Na Li Ca Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au

Single Replacement K Li F Br

Will It React? • Virtual Lab

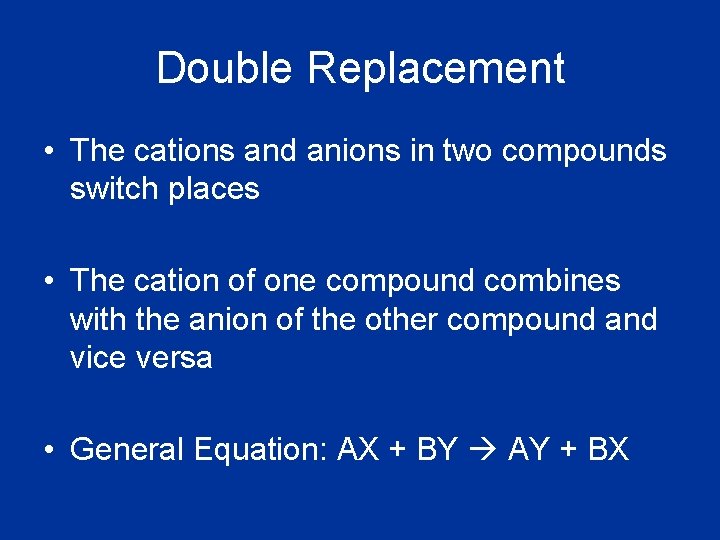
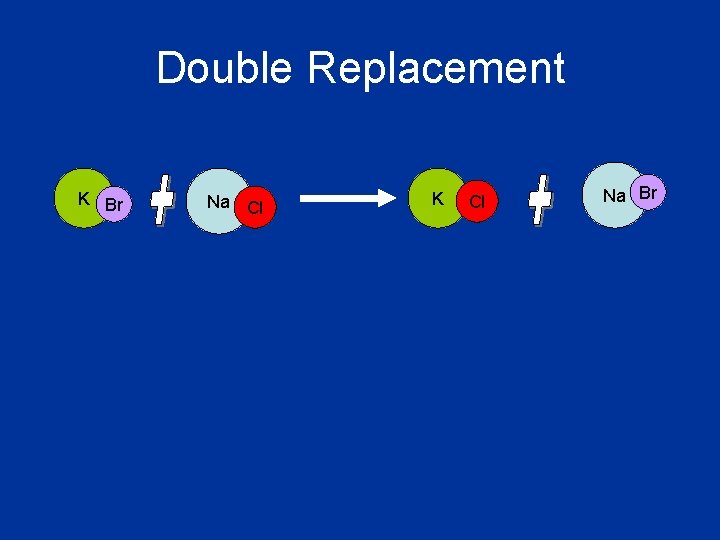
Double Replacement • The cations and anions in two compounds switch places • The cation of one compound combines with the anion of the other compound and vice versa • General Equation: AX + BY AY + BX

Double Replacement K Br Na Cl K Cl Na Br

Combustion • A substance reacts with oxygen • Combustion means “burn” • The complete combustion of a hydrocarbon produces carbon dioxide and water • Incomplete combustion can lead to the production of carbon monoxide

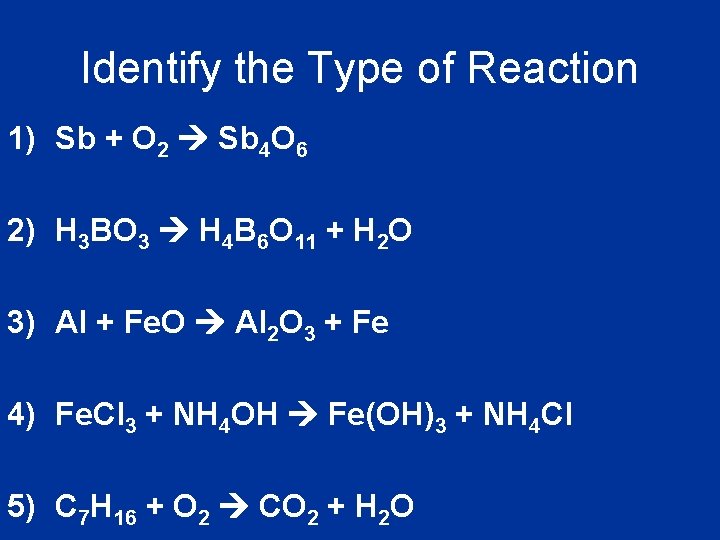
Identify the Type of Reaction 1) Sb + O 2 Sb 4 O 6 2) H 3 BO 3 H 4 B 6 O 11 + H 2 O 3) Al + Fe. O Al 2 O 3 + Fe 4) Fe. Cl 3 + NH 4 OH Fe(OH)3 + NH 4 Cl 5) C 7 H 16 + O 2 CO 2 + H 2 O