CHEMICAL REACTIONS ENZYMES CHAPTER 2 I CHEMICAL REACTIONS







- Slides: 15

CHEMICAL REACTIONS & ENZYMES CHAPTER 2

I. CHEMICAL REACTIONS • a. Chemical reaction- process that changes one set of chemicals into another • i. Changes in chemical bonds • ii. Example: CO 2 + H 2 O H 2 CO 3 • (Reactants) (Product- carbonic acid) • 1. Cells and tissues produce CO 2 to lungs via bloodstream, exhaled

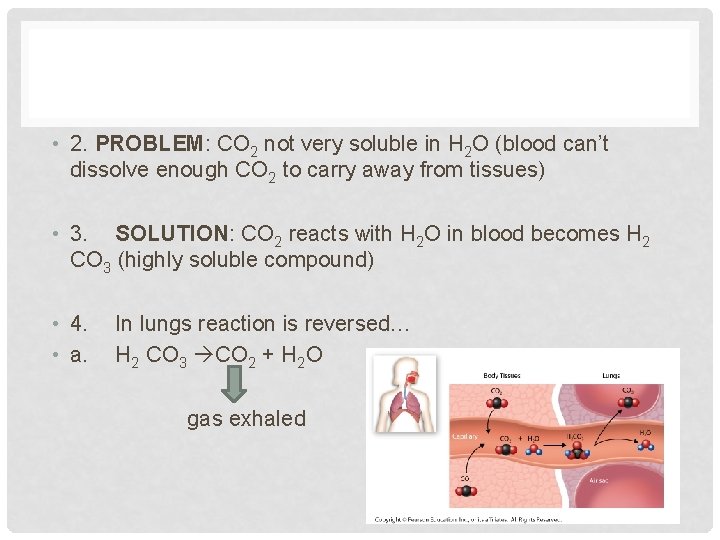
• 2. PROBLEM: CO 2 not very soluble in H 2 O (blood can’t dissolve enough CO 2 to carry away from tissues) • 3. SOLUTION: CO 2 reacts with H 2 O in blood becomes H 2 CO 3 (highly soluble compound) • 4. • a. In lungs reaction is reversed… H 2 CO 3 CO 2 + H 2 O gas exhaled

II. ENERGY IN REACTIONS • Energy is RELEASED or ABSORBED when bonds broken or formed • Exothermic- energy is released, often occur spontaneously • 2 H 2 + O 2 2 H 2 O + E • Hydrogen gas burns, or reacts with oxygen producing water vapor • Energy released as: heat, light, sound

• ii. Endothermic- energy is absorbed, won’t occur w/o energy source • 1. 2 H 2 O + E 2 H 2 + O 2 • a. Reverse of above- water changed into hydrogen gas and oxygen gas • b. Absorbs so much energy can’t occur by itself ( may need electrical source)

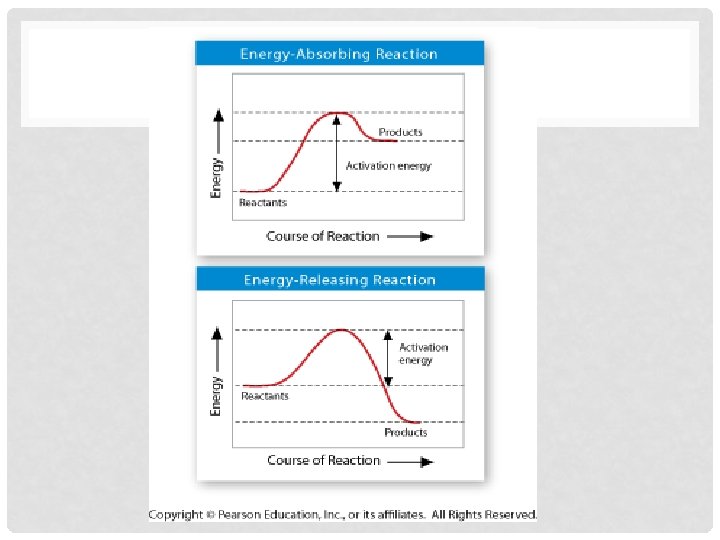
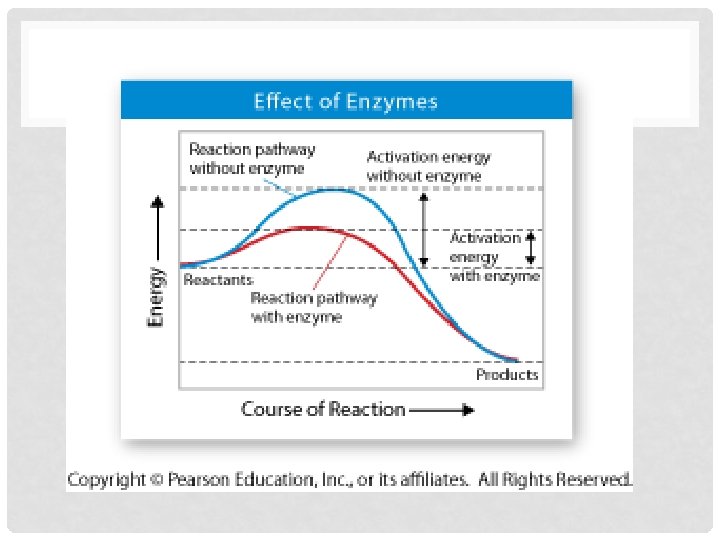
ACTIVATION ENERGY • b. Activation Energy- energy needed to get reaction started • i. See Figure 2 -20 page 51 • 1. What happens during reaction when products contain more energy than reactants? • a. Energy is absorbed • 2. What happens during reaction when products contain less energy than reactants? • a. Energy is released • 3. Which graph could represent reaction in which food is broken down for energy? • a. Energy-releasing reaction


III. ENZYMES • • a. Enzymesi. Proteins ii. biological catalysts iii. speed up rate of reactions by lowering activation energy


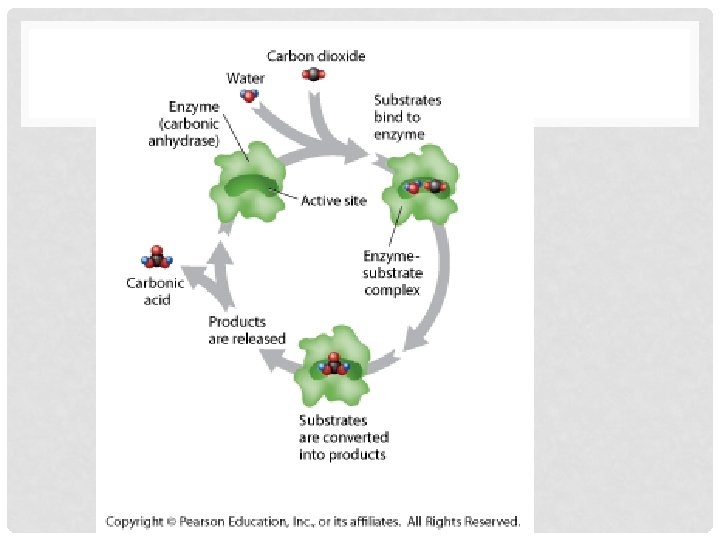
• b. Example of Enzyme importance: • i. CO 2 + H 2 O H 2 CO 3 • ii. Reaction would occur so slowly that CO 2 would build up in body faster than bloodstream could remove it • iii. Carbonic anhydrase- enzyme speeds up reaction by 10 million x’s


C. ENZYME SPECIFICITY • ii. Most enzymes only catalyze ONE reaction Name derived from reaction its catalyzes • d. How do Enzymes Work? • i. For reaction to occur reactants must collide with enough energy to break bonds and form new ones • ii. If not enough energy reactants don’t change • iii. Enzymes provide area for reactants to react together thus reducing energy needed for them to break bonds • iv. Lock and Key Model- active site has certain shape only specific substrates can bind to it

• Video- How Enzymes Work

V. THE STEPS • 1. Enzyme exists with empty active site • 2. Substrates bind to active site forming enzymesubstrate complex • 3. Substrates converted into products • 4. Products are released from active site • 5. Enzyme can be used again for another reaction (cycle)

REGULATION OF ENZYME ACTIVITY • i. Temperature- produced by human cells work best at 37ºC • 1. denaturation- if temp too high or low, enzymes changes its shape, substrates don’t fit in active site, reaction can’t occur, irreversible • ii. p. H- most work best around 7, some (stomachpepsin) work best at acidic p. H • iii. Regulatory molecules- switch enzymes “on” or “off” on as needed basis