CHEMICAL REACTIONS ENERGY TRANFER SC 5 17 CW














- Slides: 80

CHEMICAL REACTIONS & ENERGY TRANFER SC 5 -17 CW: DISCUSSES THE IMPORTANCE OF CHEMICAL REACTIONS IN THE PRODUCTION OF A RANGE OF SUBSTANCES, AND THE INFLUENCE OF SOCIETY ON THE DEVELOPMENT OF NEW MATERIALS. CW 3: Chemical reactions involve rearranging atoms to form new substances; during a chemical reaction mass is not created nor destroyed. Cw 4: Different types of chemical reactions are used to produce a range of products and can occur at different rates and involve energy transfer.

BOOKLET �This booklet forms 10% of your final assessment mark for this unit. �Marks will be awarded for: Completion of work to a high standard Ability to follow written instructions Completion of practical’s and practical reports �Marks will be deducted for: Graffiti Plagiarism Inability to follow written instructions

ATOMIC THEORY INSIDE THE ATOM �All chemicals — in other words all substances — are made up of tiny particles. These tiny particles are so small that you can't see them, even with the most powerful microscope. �Atomic theory is the scientific theory of the nature of matter. The theory states that matter is made up of small particles called atoms.

INSIDE THE ATOM An atom is the smallest part of an element that contains all the properties or characteristics of that element. An element is a substance that is made up of only one type of atom, whether those atoms exists singly or as molecules. �Prior to this theory, matter was thought to be able to be divided into any small quantity. The word atom is derived from the Greek atomos, meaning indivisible.

DEVELOPMENT OF ATOMIC THEORY �Dalton’s atomic theory 1803 All matter is made of atoms and cannot be broken down All atoms of an element are identical to each other but different from other elements Atoms are rearranged in a chemical reaction Compounds are formed when two or more different atoms join together �JJ Thompson’s discovery of the electron 1897 – Plum Pudding Model Atoms consist of a large sphere of positive charge embedded with smaller negatively charged particles. The total positive charge equals the total negative charge.

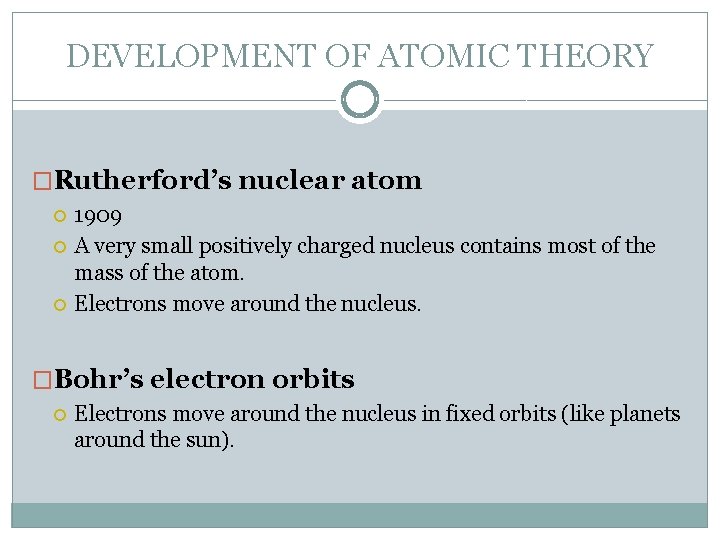
DEVELOPMENT OF ATOMIC THEORY �Rutherford’s nuclear atom 1909 A very small positively charged nucleus contains most of the mass of the atom. Electrons move around the nucleus. �Bohr’s electron orbits Electrons move around the nucleus in fixed orbits (like planets around the sun).

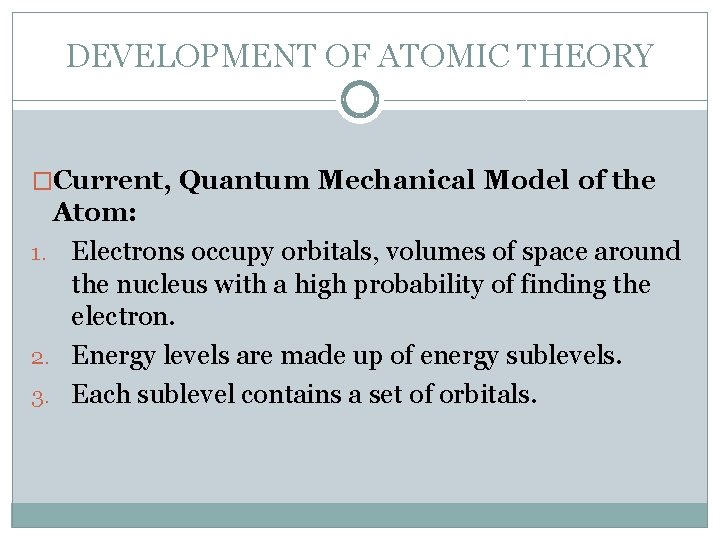
DEVELOPMENT OF ATOMIC THEORY �Current, Quantum Mechanical Model of the Atom: 1. Electrons occupy orbitals, volumes of space around the nucleus with a high probability of finding the electron. 2. Energy levels are made up of energy sublevels. 3. Each sublevel contains a set of orbitals.

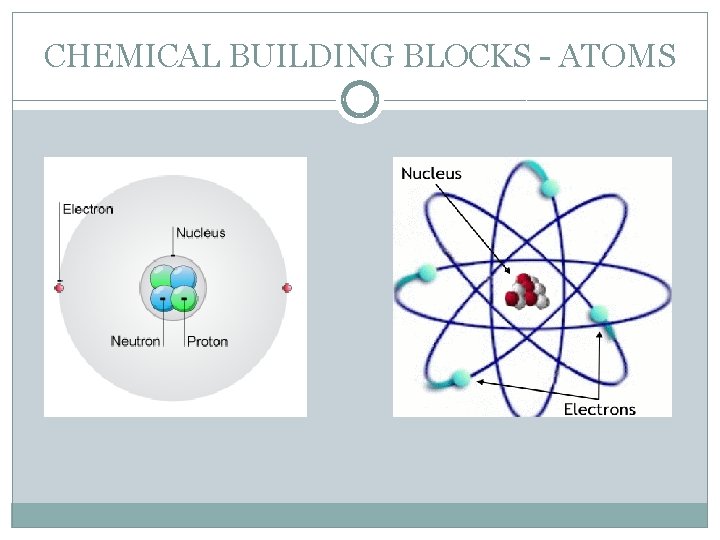
CHEMICAL BUILDING BLOCKS - ATOMS �Currently, the accepted model of an atom is that it consists of a nucleus, made up of neutrons (NEUTral/No charge) and protons (Positive), surrounded by rapidly moving electrons (negative). �The force that keeps the electrons and protons attracted to each other is called Electrostatic attraction. Since protons are positive and electrons are negative, they attract each other and keep the atom from collapsing on its self.

CHEMICAL BUILDING BLOCKS - ATOMS

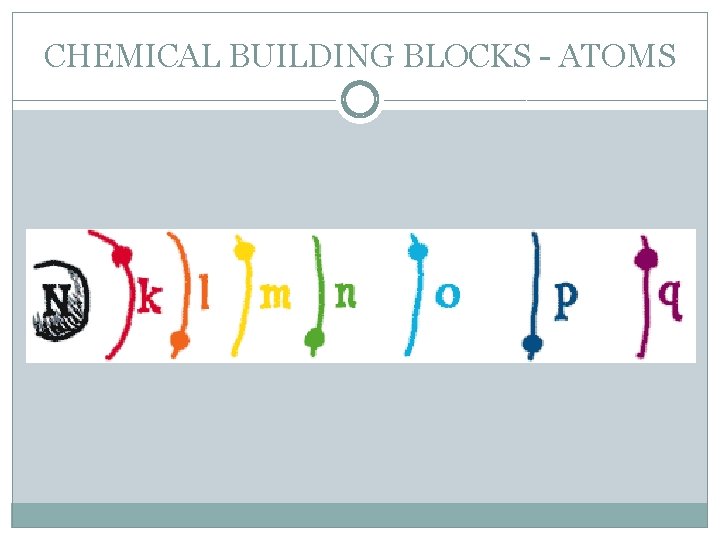
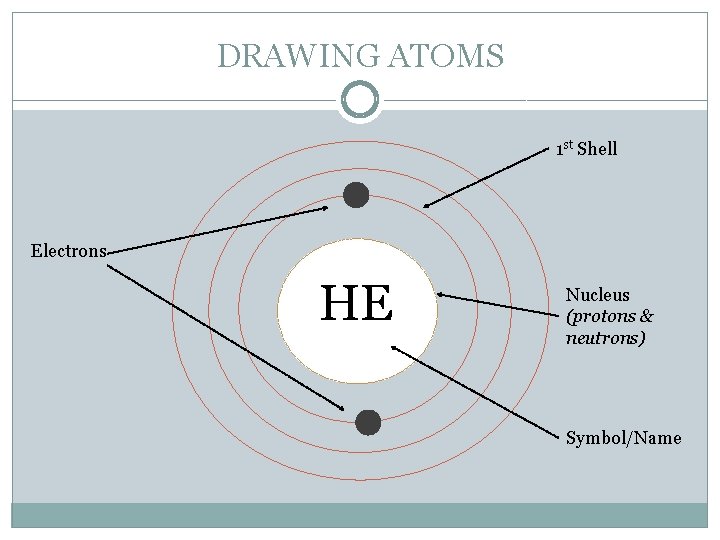
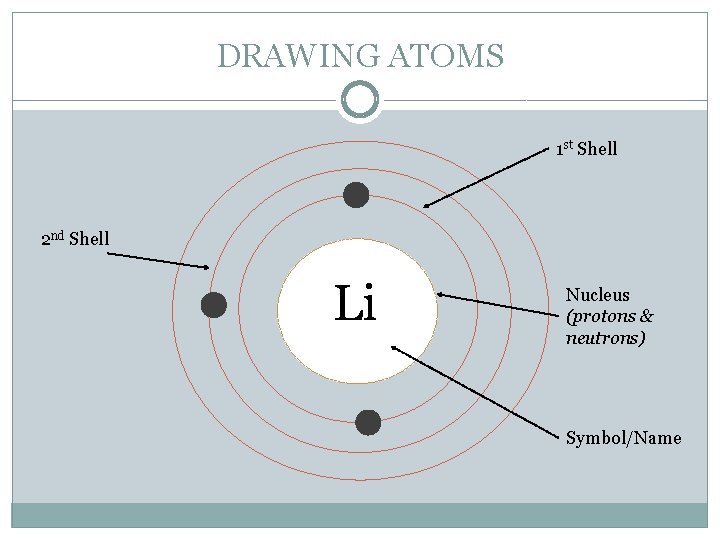
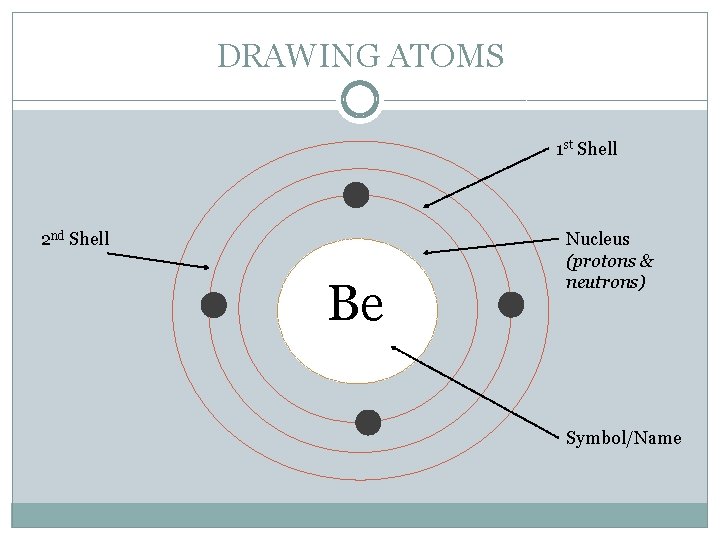
CHEMICAL BUILDING BLOCKS - ATOMS �Electrons are arranged in different shells or energy levels around the nucleus. Each shell can only hold a certain number of electrons before it becomes full. The innermost or first shell (K) can hold a maximum of two electrons. The second shell (L) a maximum of eight. The third shell (M) a maximum of eight.

CHEMICAL BUILDING BLOCKS - ATOMS

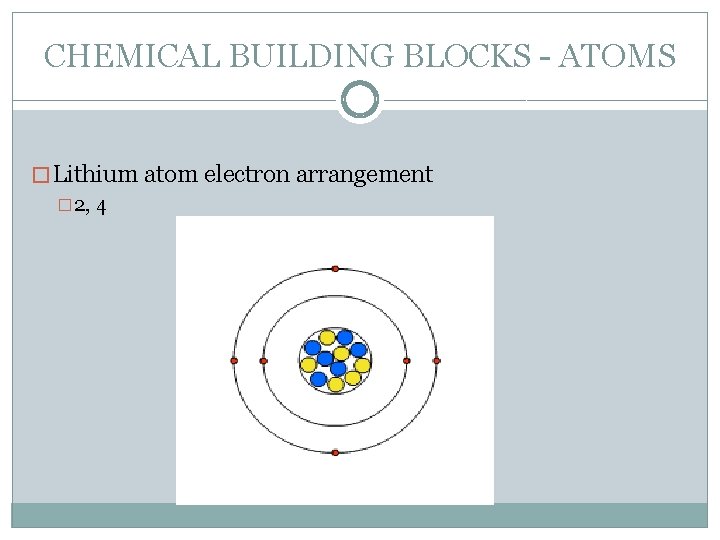
CHEMICAL BUILDING BLOCKS - ATOMS � Lithium atom electron arrangement � 2, 4

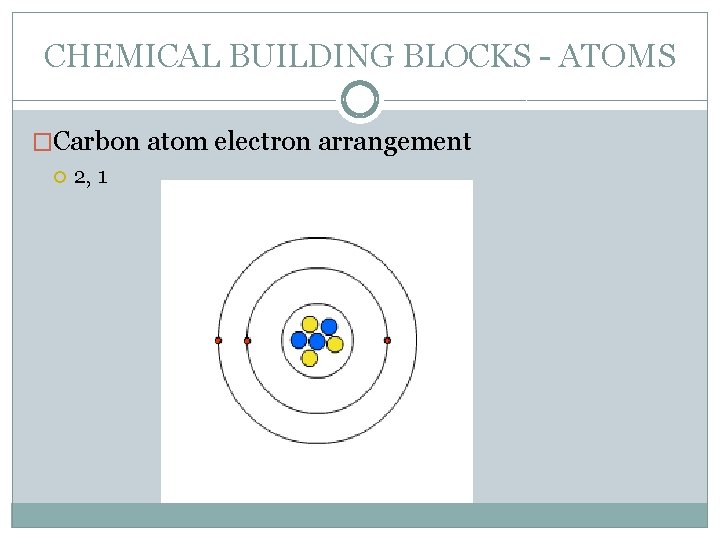
CHEMICAL BUILDING BLOCKS - ATOMS �Carbon atom electron arrangement 2, 1

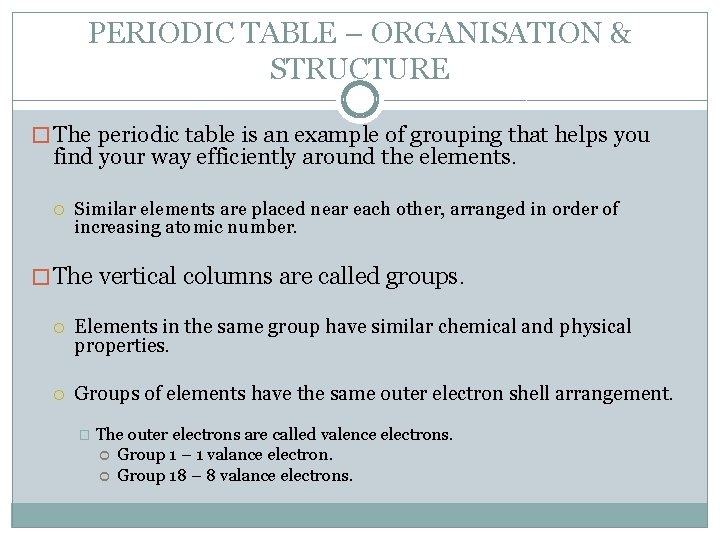
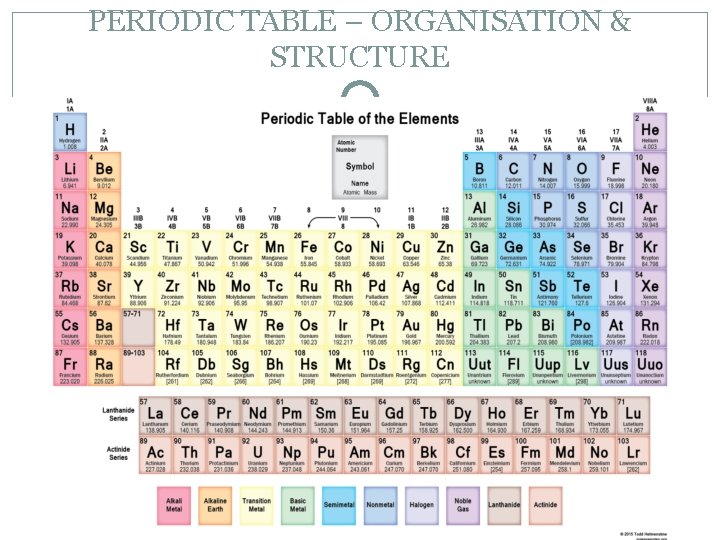
PERIODIC TABLE – ORGANISATION & STRUCTURE � The periodic table is an example of grouping that helps you find your way efficiently around the elements. Similar elements are placed near each other, arranged in order of increasing atomic number. � The vertical columns are called groups. Elements in the same group have similar chemical and physical properties. Groups of elements have the same outer electron shell arrangement. � The outer electrons are called valence electrons. Group 1 – 1 valance electron. Group 18 – 8 valance electrons.

PERIODIC TABLE – ORGANISATION & STRUCTURE

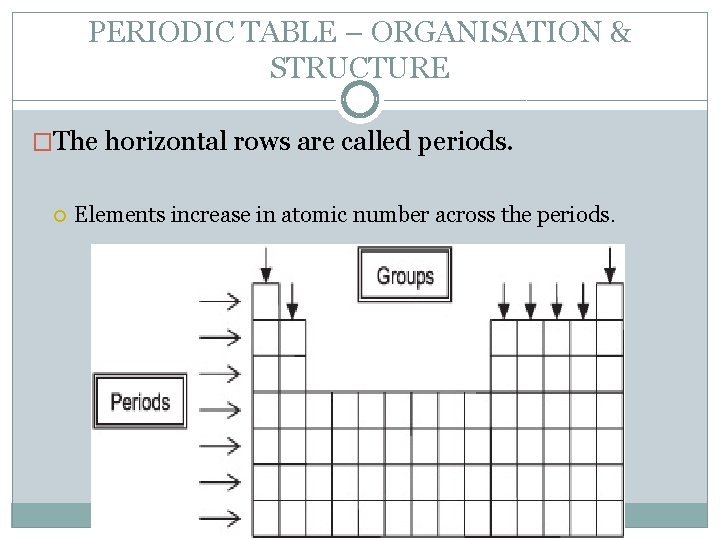
PERIODIC TABLE – ORGANISATION & STRUCTURE �The horizontal rows are called periods. Elements increase in atomic number across the periods.

PERIODIC TABLE – ORGANISATION & STRUCTURE

PERIODIC TABLE – ORGANISATION & STRUCTURE �The atomic number (top number) is the number of protons in an atom. Atoms with the same atomic number have identical chemical properties. Because atoms are electrically neutral, the number of protons in an atom is the same as the number of electrons. �The mass number (bottom number) is the total number of protons and neutrons in an atom. The number of neutrons in an atom can be calculated by subtracting the atomic number from the mass number.

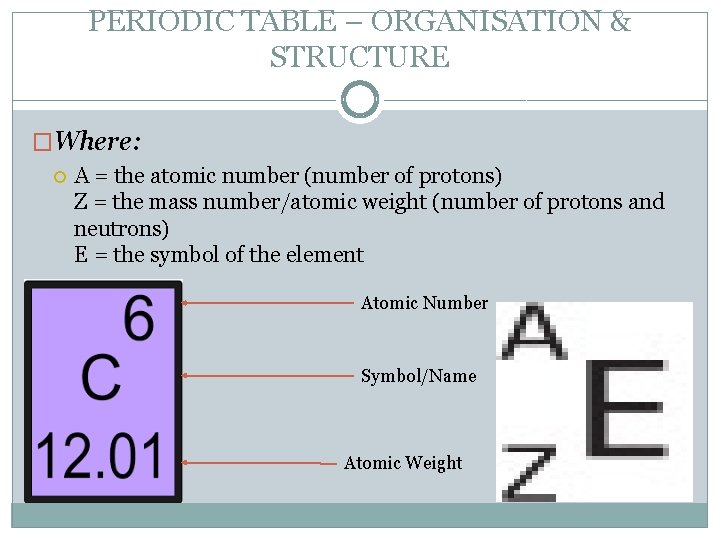
PERIODIC TABLE – ORGANISATION & STRUCTURE �Where: A = the atomic number (number of protons) Z = the mass number/atomic weight (number of protons and neutrons) E = the symbol of the element Atomic Number Symbol/Name Atomic Weight

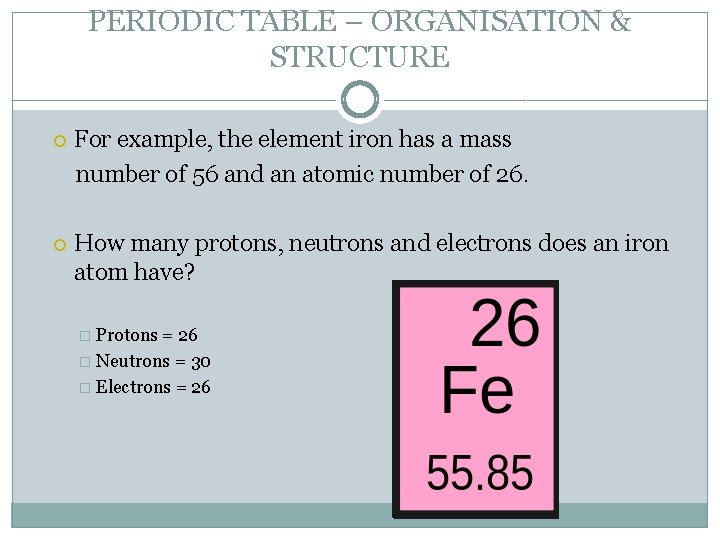
PERIODIC TABLE – ORGANISATION & STRUCTURE For example, the element iron has a mass number of 56 and an atomic number of 26. How many protons, neutrons and electrons does an iron atom have? � Protons = 26 � Neutrons = 30 � Electrons = 26

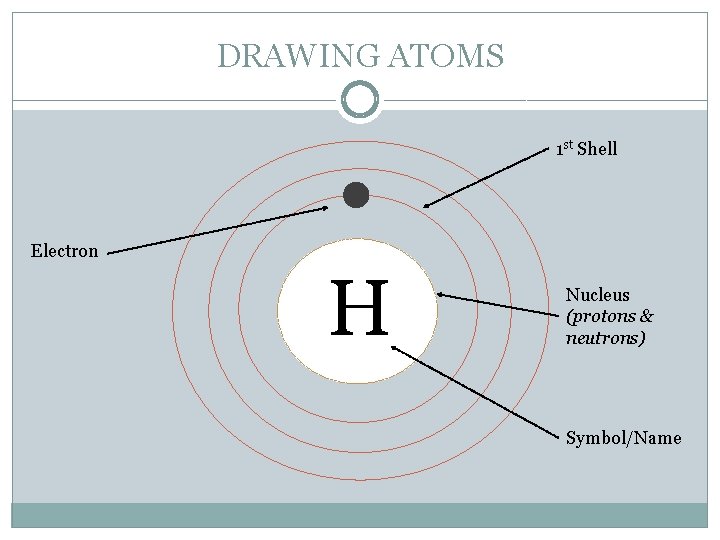
DRAWING ATOMS 1 st Shell Electron H Nucleus (protons & neutrons) Symbol/Name

DRAWING ATOMS 1 st Shell Electrons HE Nucleus (protons & neutrons) Symbol/Name

DRAWING ATOMS 1 st Shell 2 nd Shell Li Nucleus (protons & neutrons) Symbol/Name

DRAWING ATOMS 1 st Shell 2 nd Shell Be Nucleus (protons & neutrons) Symbol/Name

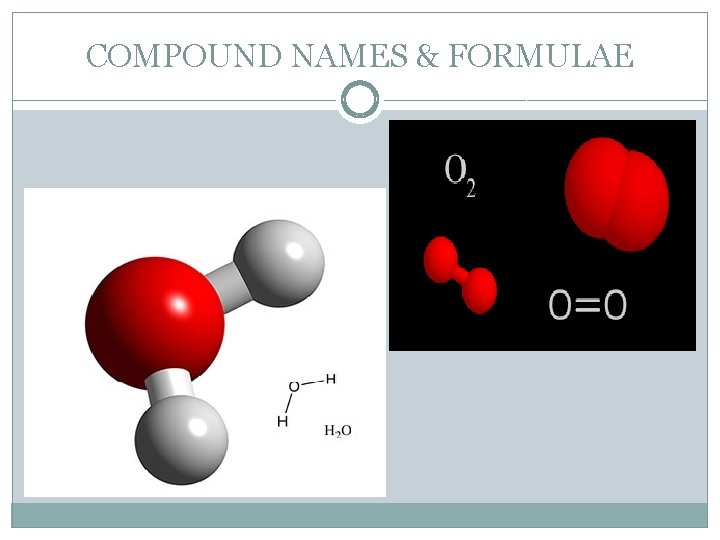
COMPOUND NAMES & FORMULAE �Most of the chemicals used in the laboratory are identified by both a name and a formula. �Chemical compounds are formed when elements are joined by chemical bonds. These bonds are so strong that the compound behaves like a single substance. A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds.

COMPOUND NAMES & FORMULAE

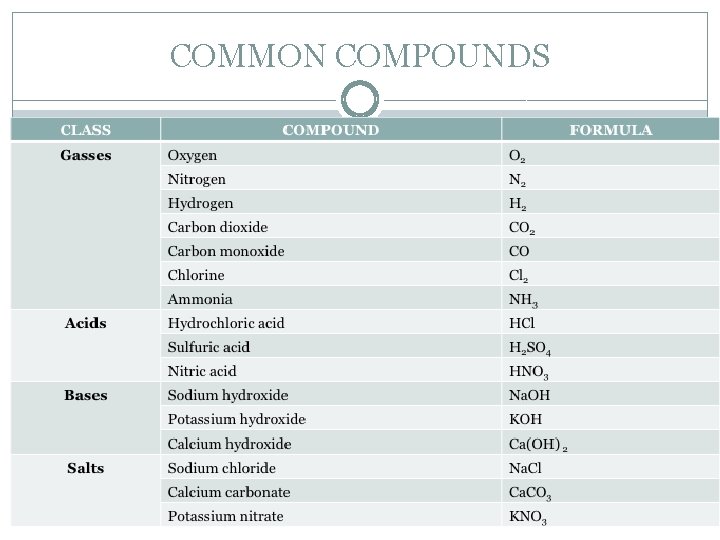
COMMON COMPOUNDS

COMPOUND NAMES & FORMULAE �To read a chemical formula, it is necessary to note the number after the chemical symbol – the subscript (small number). This number denotes the number of atoms of that element in the compound. �If a symbol has no number in the subscript, only one atom of that element is present in that compound.

NAMING COMPOUNDS �The process of naming compounds is just a set of rules. �Chemical compounds can be divided into two basic types - ionic and covalent. Ionic compounds are easily recognised because they contain a metal and a non-metal – electrons are transferred. Covalent compounds are those that contain only non-metals – electrons are shared.

IONIC COMPOUNDS �The names for ionic compounds are very simple. The first part of the name is simply the name of the metal element. The second part of the name is the name of the non-metal element, with the ending changed to the suffix –ide.

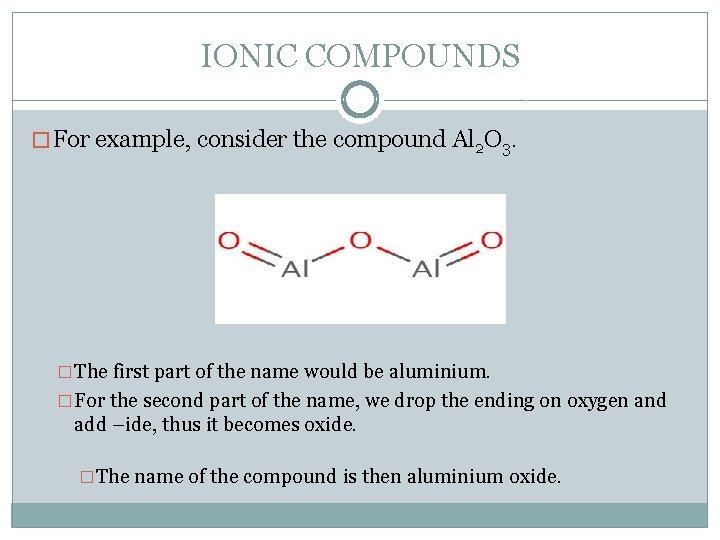
IONIC COMPOUNDS � For example, consider the compound Al 2 O 3. �The first part of the name would be aluminium. �For the second part of the name, we drop the ending on oxygen and add –ide, thus it becomes oxide. �The name of the compound is then aluminium oxide.

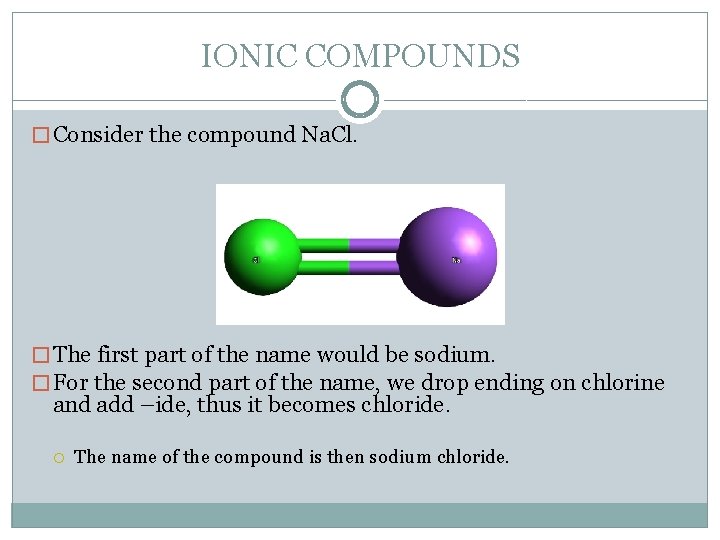
IONIC COMPOUNDS � Consider the compound Na. Cl. � The first part of the name would be sodium. � For the second part of the name, we drop ending on chlorine and add –ide, thus it becomes chloride. The name of the compound is then sodium chloride.

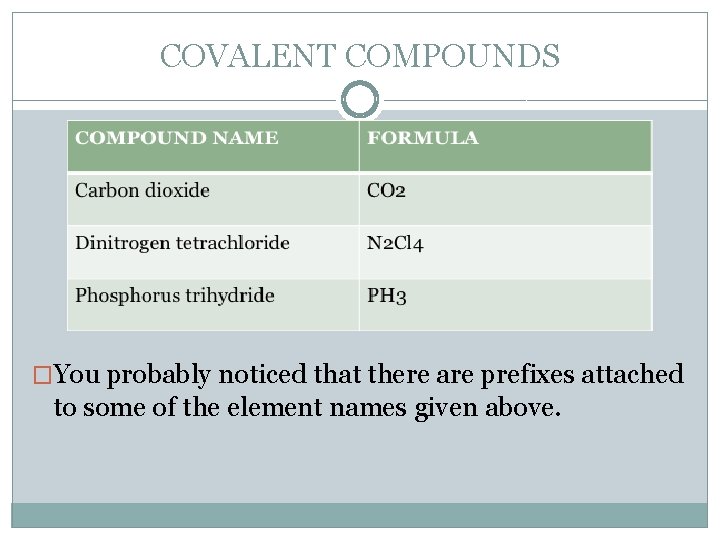
COVALENT COMPOUNDS �Before we get started naming, let's look at some simple covalent compound names and their formulas at the same time. Look at the name, and then look at the formula. Does the compound name give you any clues about the formula?

COVALENT COMPOUNDS �You probably noticed that there are prefixes attached to some of the element names given above.

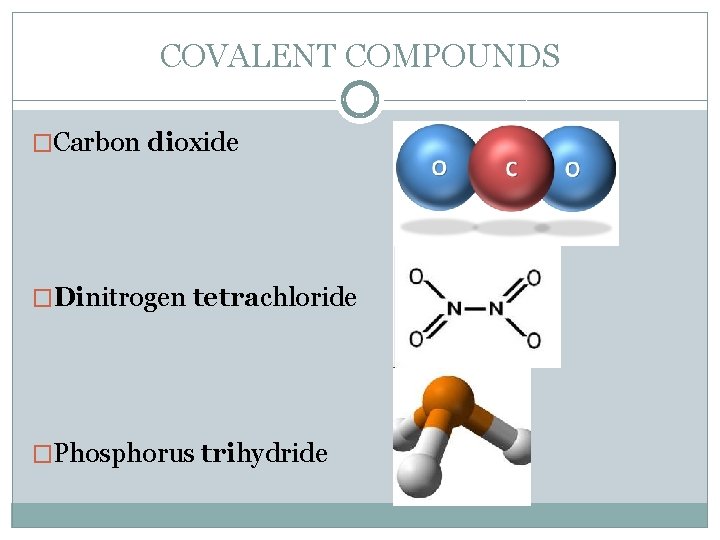
COVALENT COMPOUNDS �Carbon dioxide �Dinitrogen tetrachloride �Phosphorus trihydride

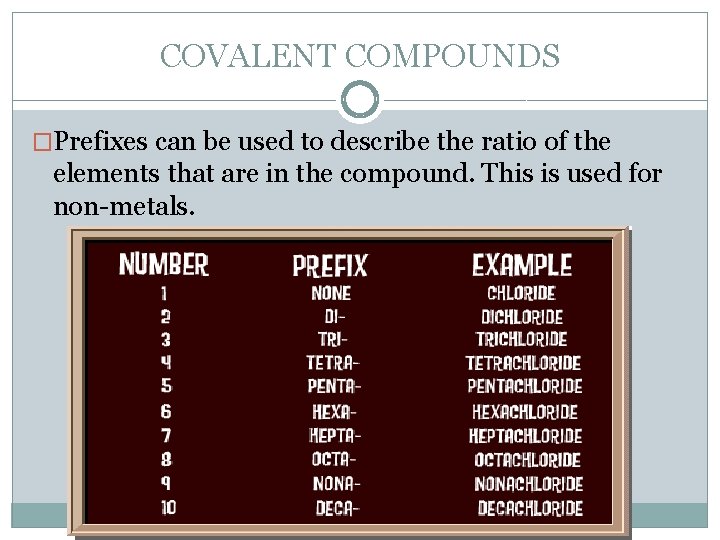
COVALENT COMPOUNDS �Prefixes can be used to describe the ratio of the elements that are in the compound. This is used for non-metals.

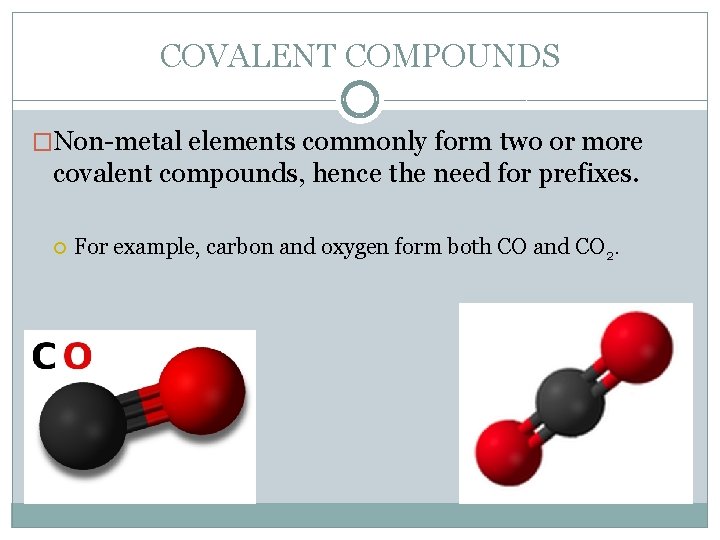
COVALENT COMPOUNDS �Non-metal elements commonly form two or more covalent compounds, hence the need for prefixes. For example, carbon and oxygen form both CO and CO 2.

COVALENT COMPOUNDS They can’t both be called carbon oxide, so we have to have a way of telling them apart. One of these is a highly toxic gas, while the other is used to carbonate beverages, so you certainly wouldn’t want to mix them up! �To distinguish covalent compounds with more than one formula, prefixes are used to indicate how many atoms of each element are present.

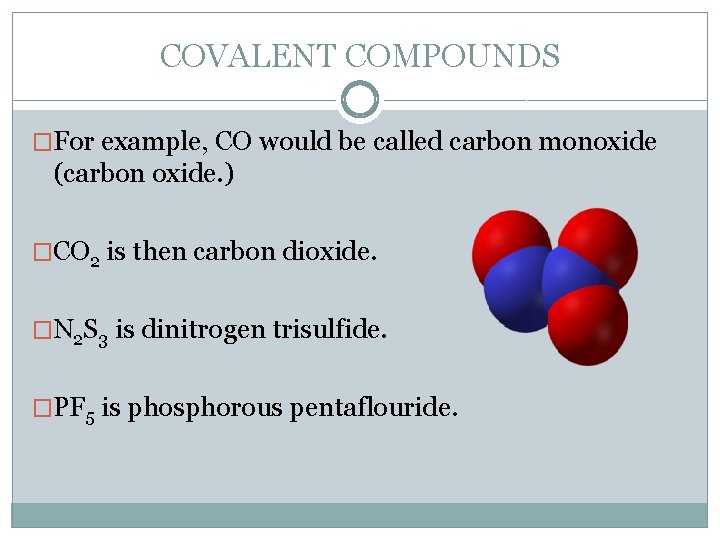
COVALENT COMPOUNDS �For example, CO would be called carbon monoxide (carbon oxide. ) �CO 2 is then carbon dioxide. �N 2 S 3 is dinitrogen trisulfide. �PF 5 is phosphorous pentaflouride.

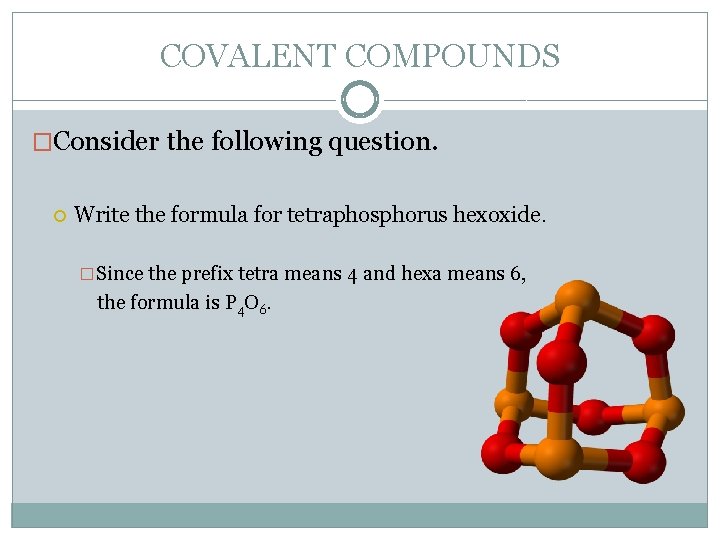
COVALENT COMPOUNDS �Consider the following question. Write the formula for tetraphosphorus hexoxide. �Since the prefix tetra means 4 and hexa means 6, the formula is P 4 O 6.

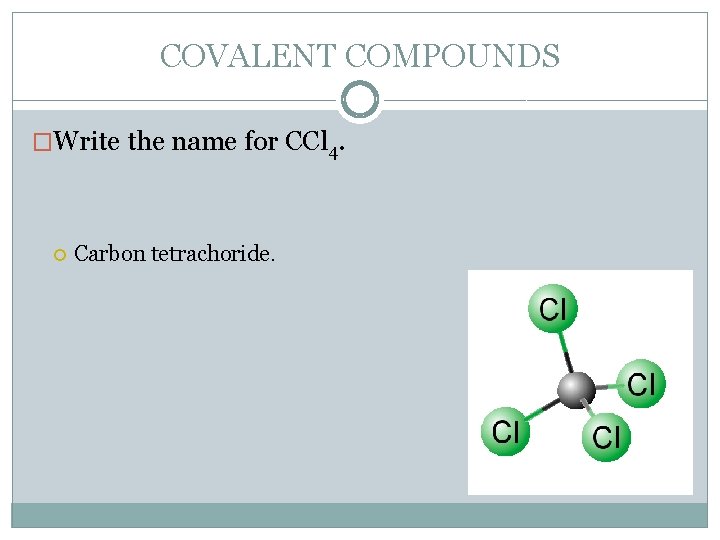
COVALENT COMPOUNDS �Write the name for CCl 4. Carbon tetrachoride.

CHEMICAL REACTIONS �Previously, we have discussed the chemical names and formulae of a range of common compounds. Now we are going to discuss chemical reactions, reactants and products in the form of chemical equations using this knowledge. �The chemical formulae of compounds obey the Law of Constant Proportions. Every compound has a fixed relative number of each type of atom.

CHEMICAL REACTIONS �For example: Water – H 2 O – always has 2 hydrogen atoms for 1 oxygen atom. Sodium – Na. Cl – always has 1 sodium atom for 1 chlorine atom.

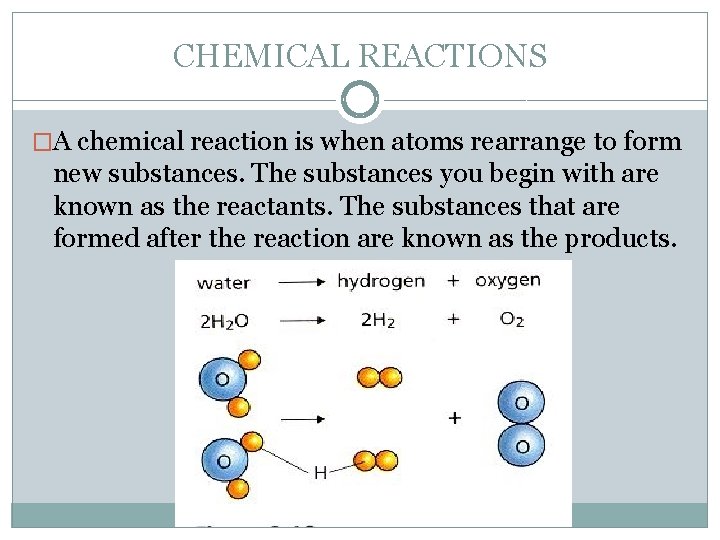
CHEMICAL REACTIONS �A chemical reaction is when atoms rearrange to form new substances. The substances you begin with are known as the reactants. The substances that are formed after the reaction are known as the products.

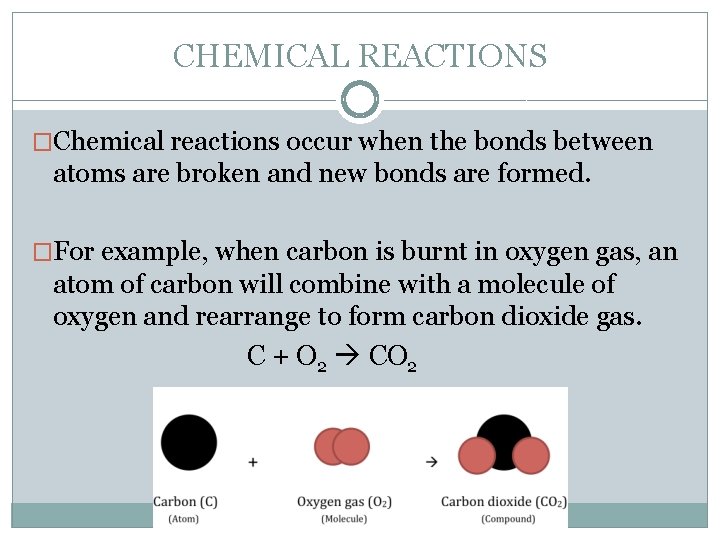
CHEMICAL REACTIONS �Chemical reactions occur when the bonds between atoms are broken and new bonds are formed. �For example, when carbon is burnt in oxygen gas, an atom of carbon will combine with a molecule of oxygen and rearrange to form carbon dioxide gas. C + O 2 CO 2

CHEMICAL REACTIONS � Once a new substance has been formed there are observable changes: A change in temperature. A change in colour. Formation of a precipitate. Formation of a gas. � So when you stick some sherbet in your mouth and you feel it fizzing on your tongue, or smell the exhaust fumes from a passing car, you are observing evidence that a chemical reaction has taken place. We will talk more about this later in the unit when we make sherbet to demonstrate exothermic reactions.

CHEMICAL REACTIONS �During a chemical reaction, the atoms in the reactants rearrange to form the products. The atoms can only rearrange, they cannot appear, disappear or change type. Law of Conservation of Mass.

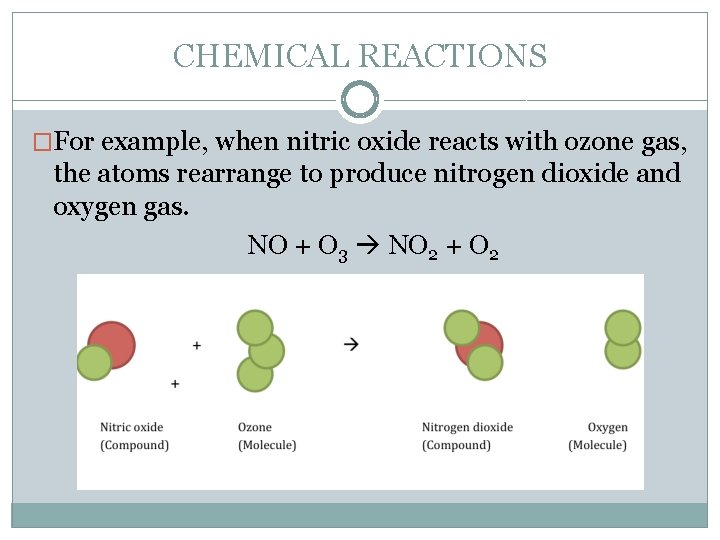
CHEMICAL REACTIONS �For example, when nitric oxide reacts with ozone gas, the atoms rearrange to produce nitrogen dioxide and oxygen gas. NO + O 3 NO 2 + O 2

CHEMICAL REACTIONS �In this chemical reaction, two reactants (nitric oxide and ozone gas) form two products (nitrogen dioxide and oxygen gas). In the reactants, there is one nitrogen atom and four oxygen atoms. The product molecules contain one nitrogen atom and four oxygen atoms. No new atoms were created and no atoms were destroyed. The chemical reaction simply rearranged the nitrogen and oxygen atoms.

CHEMICAL REACTIONS

CHEMICAL REACTIONS �Using chemical equations allows describing chemical reactions to be uniform. As we can see above, the basic chemical equation is: Reactants Products

WORD EQUATIONS �When hydrochloric acid (HCl) is mixed with sodium hydroxide (Na. OH), they combine to form a solution of sodium chloride (Na. Cl) and water (H 2 O). To describe this chemical reaction with a word equation, we write: Hydrochloric acid + Sodium hydroxide Sodium chloride + Water

WORD EQUATIONS �Construct the following into a word equation. When natural gas, methane (CH 4), is burnt in oxygen gas (O 2), it produces carbon dioxide (CO 2) and water (H 2 O). Methane + Oxygen gas Carbon dioxide + Water

FORMULA EQUATIONS �In a formula equation, the chemical name for a reactant or product is replaced with its chemical formula. �In the case of hydrochloric acid (HCl) reacting with sodium hydroxide (Na. OH) to form sodium chloride (Na. Cl) and water (H 2 O), the formula equation is simply: HCl + Na. Oh Na. Cl + H 2 O

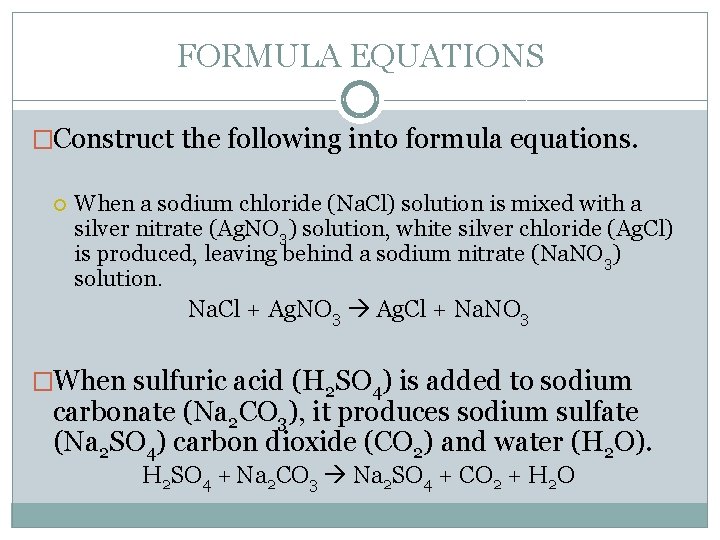
FORMULA EQUATIONS �Construct the following into formula equations. When a sodium chloride (Na. Cl) solution is mixed with a silver nitrate (Ag. NO 3) solution, white silver chloride (Ag. Cl) is produced, leaving behind a sodium nitrate (Na. NO 3) solution. Na. Cl + Ag. NO 3 Ag. Cl + Na. NO 3 �When sulfuric acid (H 2 SO 4) is added to sodium carbonate (Na 2 CO 3), it produces sodium sulfate (Na 2 SO 4) carbon dioxide (CO 2) and water (H 2 O). H 2 SO 4 + Na 2 CO 3 Na 2 SO 4 + CO 2 + H 2 O

BALANCING EQUATIONS REMEMBER: �During a chemical reaction mass is not created or destroyed. �Write a word equation and formulae equation for each of the six reactions listed. See the tables for the correct formulae and follow the game rules.

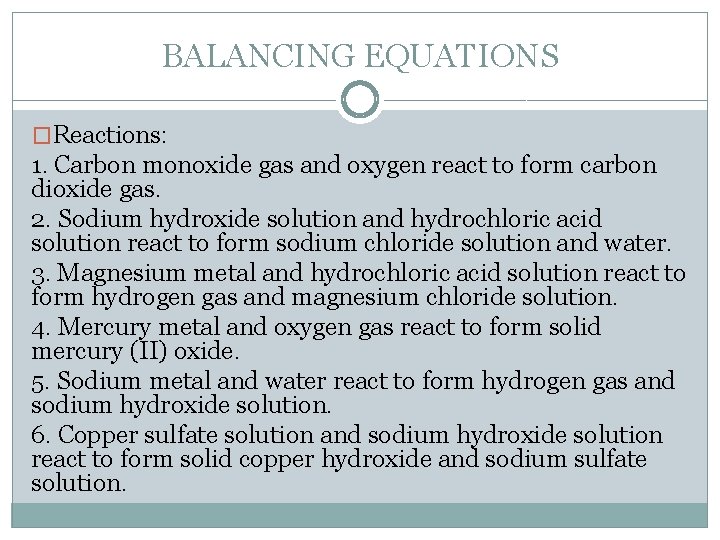
BALANCING EQUATIONS �Reactions: 1. Carbon monoxide gas and oxygen react to form carbon dioxide gas. 2. Sodium hydroxide solution and hydrochloric acid solution react to form sodium chloride solution and water. 3. Magnesium metal and hydrochloric acid solution react to form hydrogen gas and magnesium chloride solution. 4. Mercury metal and oxygen gas react to form solid mercury (II) oxide. 5. Sodium metal and water react to form hydrogen gas and sodium hydroxide solution. 6. Copper sulfate solution and sodium hydroxide solution react to form solid copper hydroxide and sodium sulfate solution.

MENTOS FOUNTAIN �What you need: 1 packet of Mentos lollies 2 L bottle of soft drink Blu-tack �What to do: Place one bottle in the fridge. Leave the other soft drink bottle at room temperature. Remove the lollies from the wrapper and stick them together with Blu-tack so they sit in a straight line. Place the bottle of soft drink on a flat surface outside. Quickly drop in the stack of Mentos and stand back!

MENTOS FOUNTAIN �What is happening: Soft drinks are mostly sugar, water and dissolved carbon dioxide. The carbon dioxide is held in the drink by strong chemical bonds between water molecules. The water acts like a net, holding each molecule of carbon dioxide in the soft drink so the molecules of carbon dioxide can’t join together to form bubbles of gas. This is why soft drinks don’t go flat as soon as you open the bottle! The surface of each Mentos has many tiny bumps and gaps which can act as ‘nucleation sites. ’ Nucleation sites are places where nucleation can occur quickly and easily. Nucleation is a physical reaction and is the process by which a substance changes its physical state in a small area.

MENTOS FOUNTAIN When a Mentos lolly is dropped into the soft drink, tiny molecules of dissolved carbon dioxide rapidly clump together at the nucleation sites, forming bubbles. Almost immediately, these bubbles rapidly float to the surface, exploding into the sky. Meanwhile, chemicals in the Mentos and the soft drink may also release the dissolved carbon dioxide through chemical reacions. Mentos contain a substance called gum arabic which is theorised to break the surface tension of the drink allowing carbon dioxide gas to bubble up and escape. Some people say it’s caused by a chemical reaction, while others say it’s caused by a physical reaction.

TYPES OF CHEMICAL REACTIONS �Countless chemical reactions take place everyday. They can be classified in various ways, whether they absorb or release energy, the nature of the reactants or products as well as the way the atoms rearrange themselves.

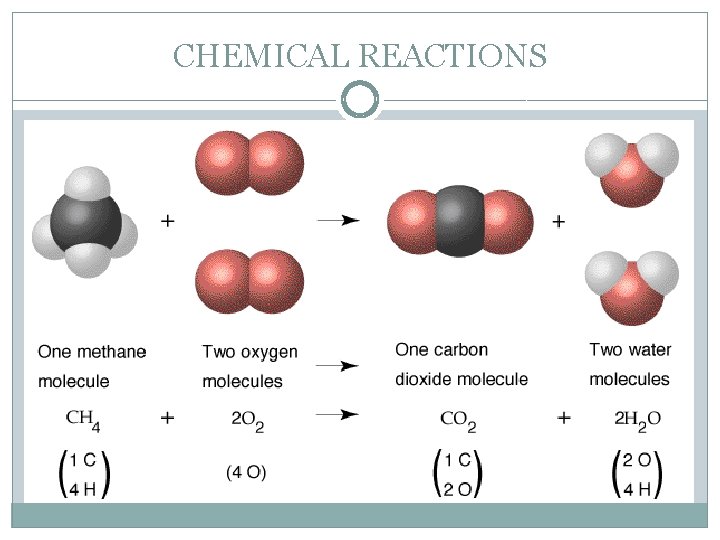
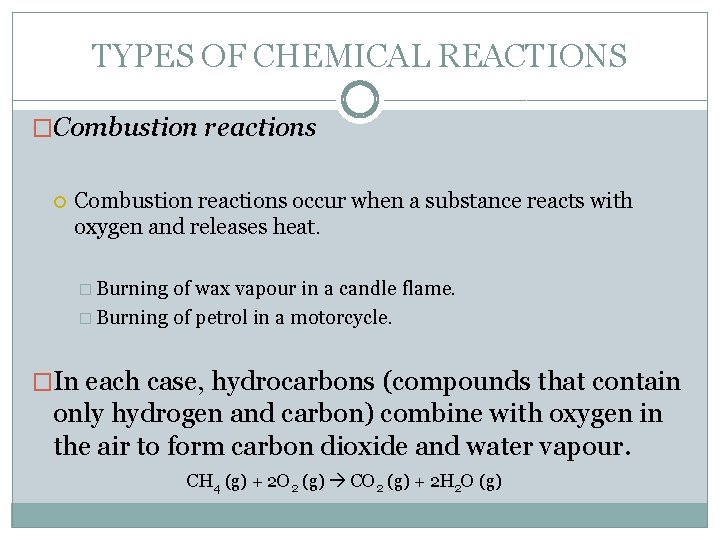
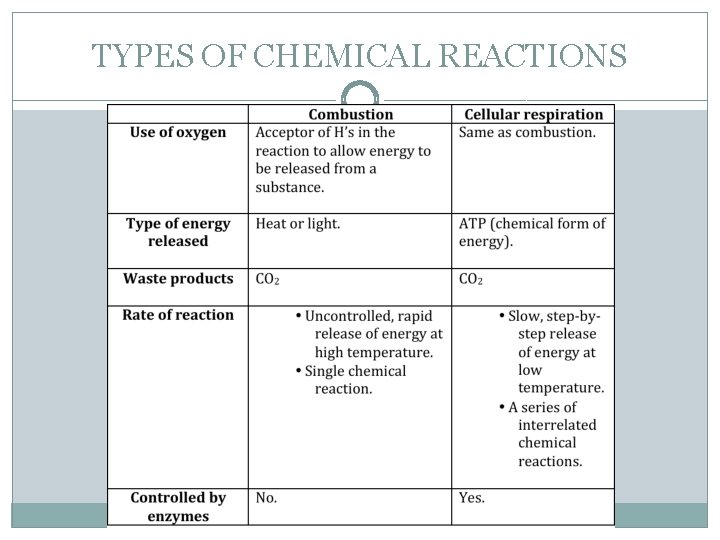
TYPES OF CHEMICAL REACTIONS �Combustion reactions occur when a substance reacts with oxygen and releases heat. � Burning of wax vapour in a candle flame. � Burning of petrol in a motorcycle. �In each case, hydrocarbons (compounds that contain only hydrogen and carbon) combine with oxygen in the air to form carbon dioxide and water vapour. CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g)

TYPES OF CHEMICAL REACTIONS �Oxygen gas is always needed for combustion to occur. For this reason, combustion reactions can also be classified as a type of oxidation reaction. �The above reaction of methane gas and oxygen gas is known as complete combustion. This is because there is no limit on the oxygen (fuel) supply and this produces a clean reaction.

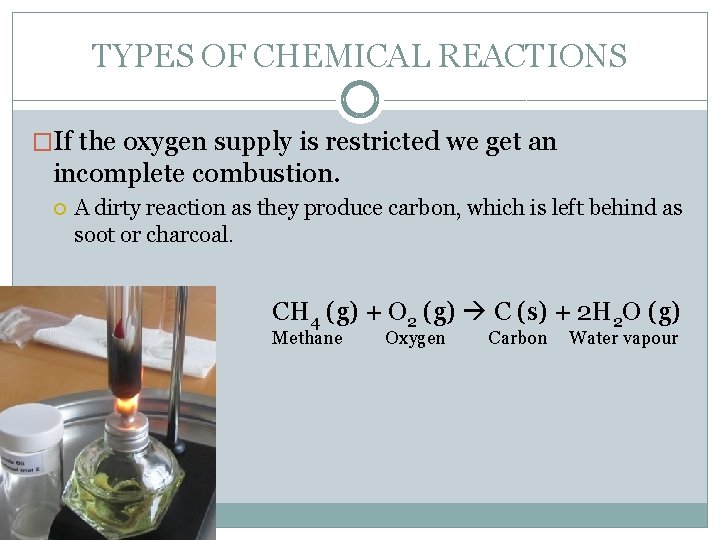
TYPES OF CHEMICAL REACTIONS �If the oxygen supply is restricted we get an incomplete combustion. A dirty reaction as they produce carbon, which is left behind as soot or charcoal. CH 4 (g) + O 2 (g) C (s) + 2 H 2 O (g) Methane Oxygen Carbon Water vapour

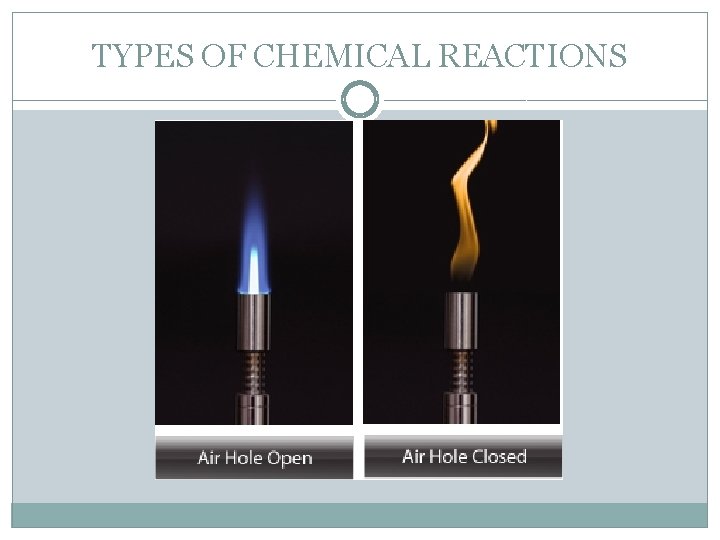
TYPES OF CHEMICAL REACTIONS �A Bunsen burner displays both complete and incomplete combustion. An open air hole allows a direct flow of oxygen and the resulting flame is hot, clean and blue – complete combustion. If you limit the flow of oxygen by slightly closing the air hole then a cooler, yellow flame is produced – incomplete combustion.

TYPES OF CHEMICAL REACTIONS

TYPES OF CHEMICAL REACTIONS �Combustion reactions not only take place in non- living systems, the also take place in living systems. Within your body combustion reactions occur, however, are much slower and more controlled. �The cellular respiration reaction takes place continuously in our body. For example, we eat pasta containing glucose molecules as small spirals called starch.

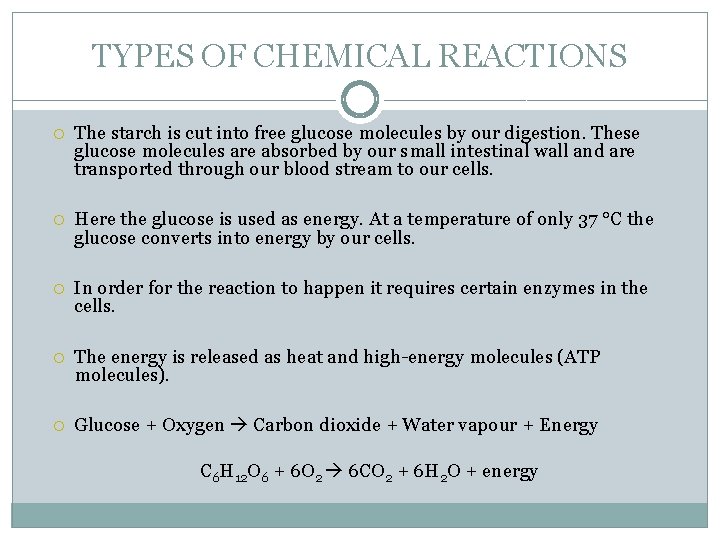
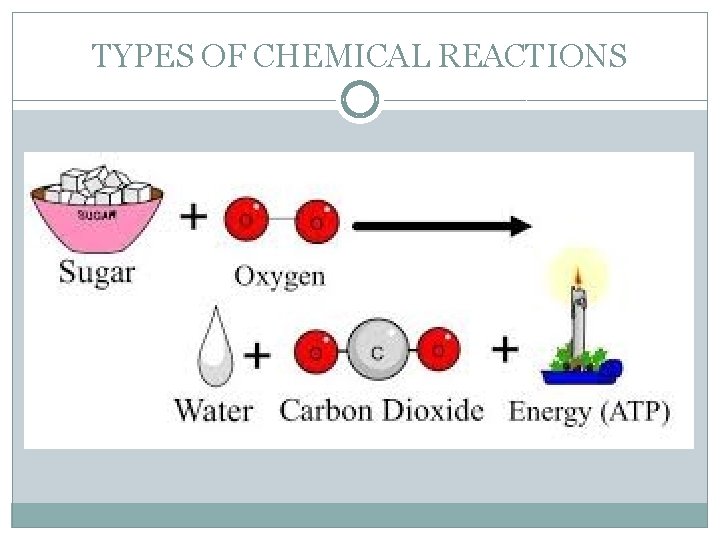
TYPES OF CHEMICAL REACTIONS The starch is cut into free glucose molecules by our digestion. These glucose molecules are absorbed by our small intestinal wall and are transported through our blood stream to our cells. Here the glucose is used as energy. At a temperature of only 37 °C the glucose converts into energy by our cells. In order for the reaction to happen it requires certain enzymes in the cells. The energy is released as heat and high-energy molecules (ATP molecules). Glucose + Oxygen Carbon dioxide + Water vapour + Energy C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + energy

TYPES OF CHEMICAL REACTIONS

TYPES OF CHEMICAL REACTIONS

TYPES OF CHEMICAL REACTIONS �The reaction of acids including metals and carbonates �Acids are corrosive substances meaning they will ‘eat away’ solid substances, they taste sour and turn blue litmus paper pink. Acids always produce salts when they react with chemical substances.

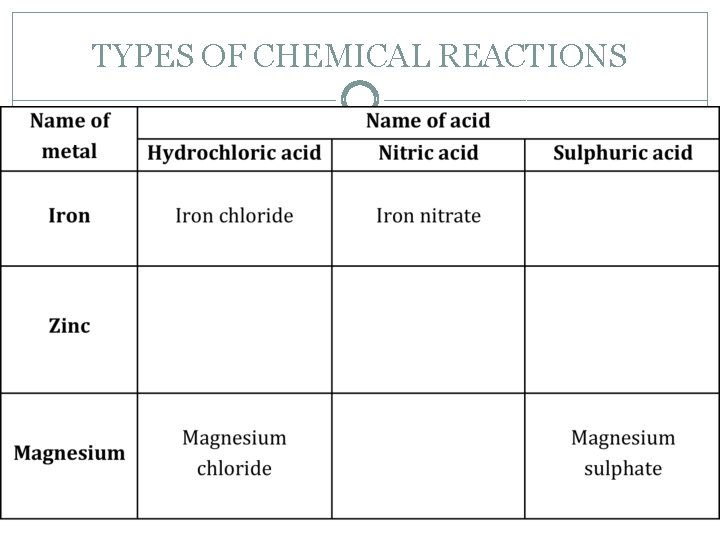
TYPES OF CHEMICAL REACTIONS The salt that is produced depends upon which acid and which metal react. Acids produce hydrogen or H+ ions in solution – p. H. The general word equation is: Acid + Metal → Salt + Hydrogen �Consider the following. (Hint – look for the pattern).

TYPES OF CHEMICAL REACTIONS

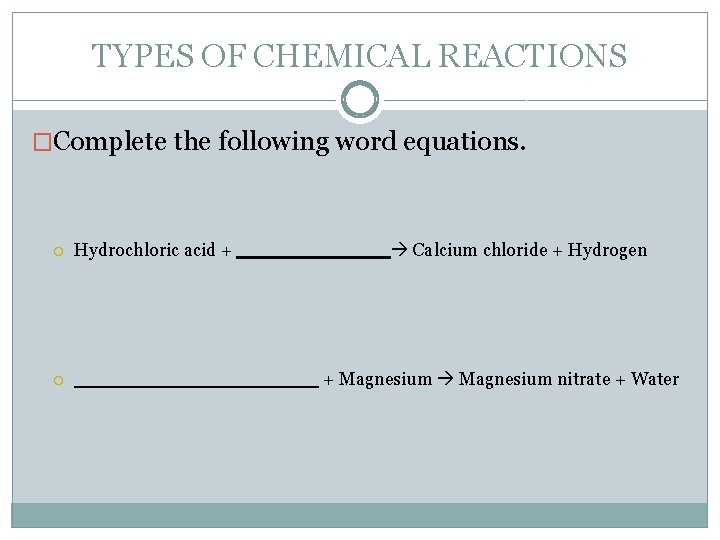
TYPES OF CHEMICAL REACTIONS �Complete the following word equations. Hydrochloric acid + + Magnesium nitrate + Water Calcium chloride + Hydrogen

TYPES OF CHEMICAL REACTIONS � The reaction of an acid and a metal is an example of a single displacement reaction. One element (metal) displaces another in a compound (acid). Metals displace the hydrogen from acids producing hydrogen gas. Magnesium + Sulphuric acid Magnesium sulphate + Hydrogen Mg + H 2 SO 4 Mg. SO 4 + H 2 The magnesium displaces the hydrogen from the acid. � It's like calling for a single substitution during a game of basketball. Mg goes on the court and H 2 comes off.

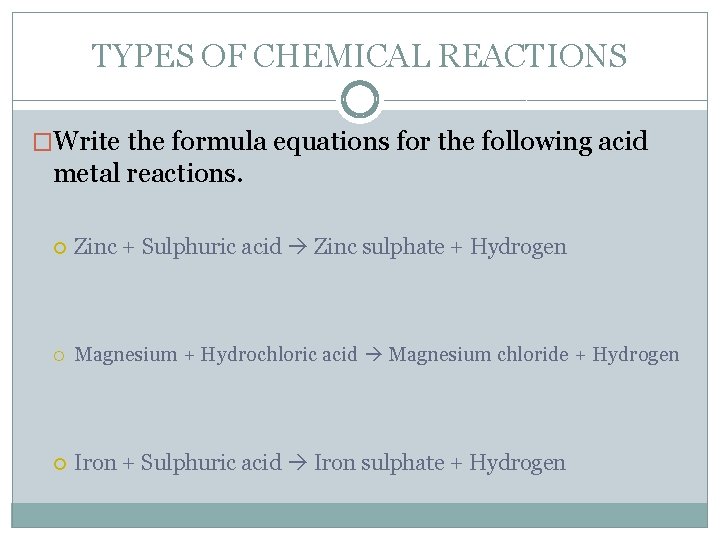
TYPES OF CHEMICAL REACTIONS �Write the formula equations for the following acid metal reactions. Zinc + Sulphuric acid Zinc sulphate + Hydrogen Magnesium + Hydrochloric acid Magnesium chloride + Hydrogen Iron + Sulphuric acid Iron sulphate + Hydrogen

TYPES OF CHEMICAL REACTIONS �Acids also react with carbonates. Carbonates are compounds that contain the CO 3 group. A common example of a carbonate is Bi-carb soda that you would find in your kitchen. Its chemical formula is Na. HCO 3.

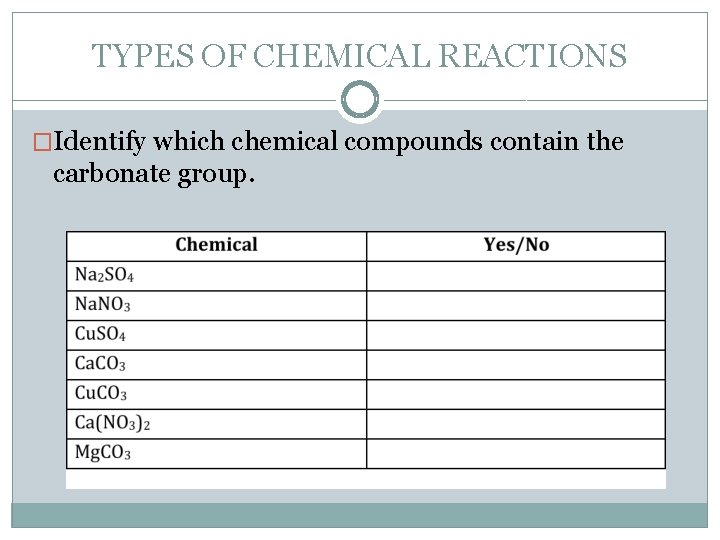
TYPES OF CHEMICAL REACTIONS �Identify which chemical compounds contain the carbonate group.

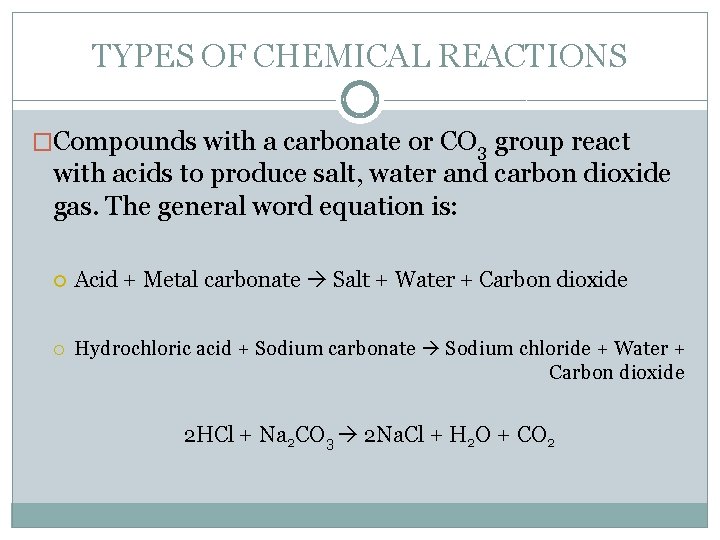
TYPES OF CHEMICAL REACTIONS �Compounds with a carbonate or CO 3 group react with acids to produce salt, water and carbon dioxide gas. The general word equation is: Acid + Metal carbonate Salt + Water + Carbon dioxide Hydrochloric acid + Sodium carbonate Sodium chloride + Water + Carbon dioxide 2 HCl + Na 2 CO 3 2 Na. Cl + H 2 O + CO 2

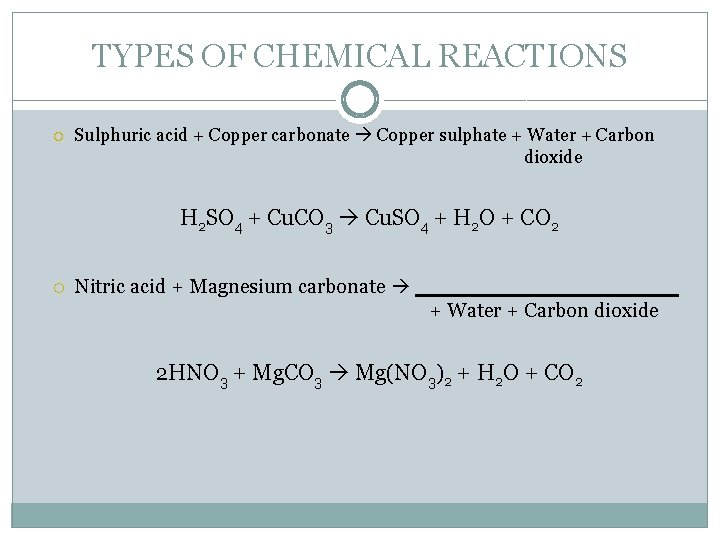
TYPES OF CHEMICAL REACTIONS Sulphuric acid + Copper carbonate Copper sulphate + Water + Carbon dioxide H 2 SO 4 + Cu. CO 3 Cu. SO 4 + H 2 O + CO 2 Nitric acid + Magnesium carbonate + Water + Carbon dioxide 2 HNO 3 + Mg. CO 3 Mg(NO 3)2 + H 2 O + CO 2