Chemical Reactions Do Now November 9 Open your

Chemical Reactions

Do Now! November 9 • Open your notes. • Write the above title • Answer the following question: – Write a chemical equation that represents the formation of table salt.
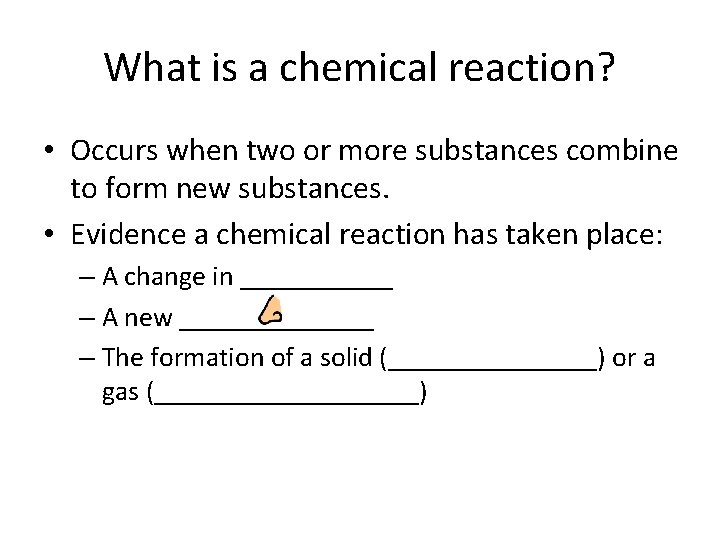
What is a chemical reaction? • Occurs when two or more substances combine to form new substances. • Evidence a chemical reaction has taken place: – A change in ______ – A new _______ – The formation of a solid (________) or a gas (__________)
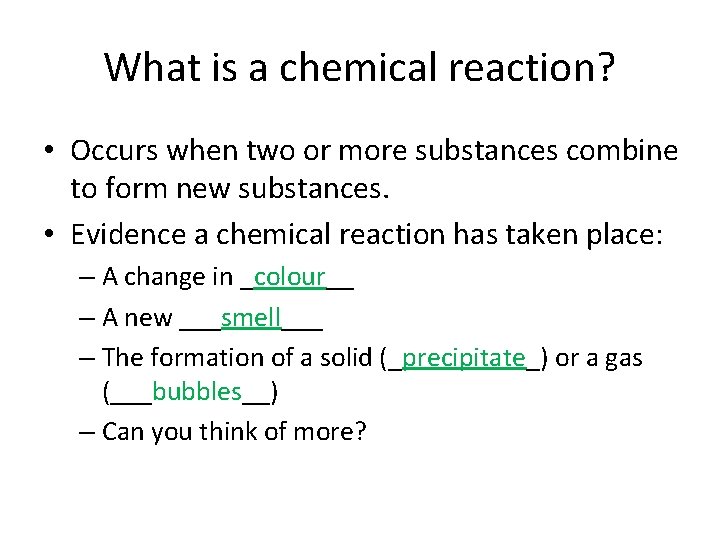
What is a chemical reaction? • Occurs when two or more substances combine to form new substances. • Evidence a chemical reaction has taken place: – A change in _colour__ – A new ___smell___ – The formation of a solid (_precipitate_) or a gas (___bubbles__) – Can you think of more?
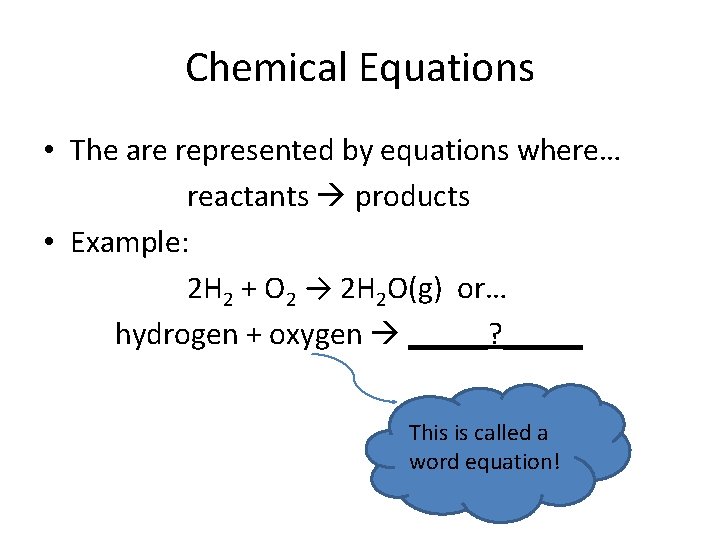
Chemical Equations • The are represented by equations where… reactants products • Example: 2 H 2 + O 2 → 2 H 2 O(g) or… hydrogen + oxygen _____? _____ This is called a word equation!
4 types of chemical reactions • • Combustion Corrosion Single replacement Double replacement

Combustion • Means to burn • The reaction: fuel + oxidant carbon dioxide + water vapour + energy A compound containing carbon and hydrogen oxygen • An example: • CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g)
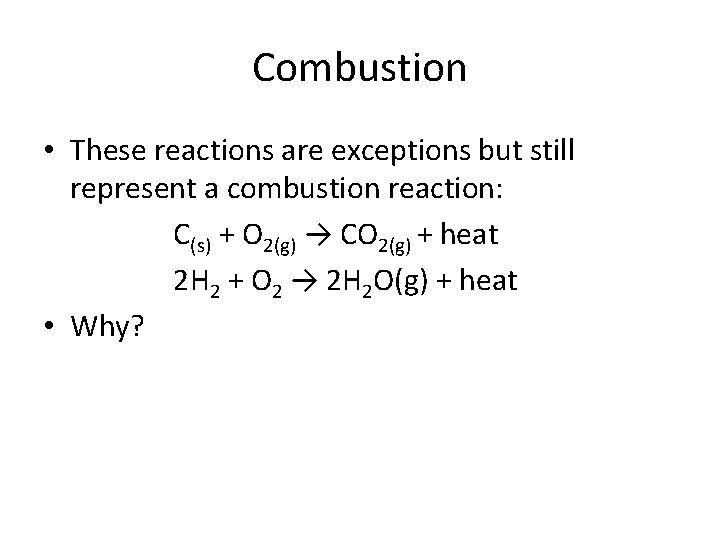
Combustion • These reactions are exceptions but still represent a combustion reaction: C(s) + O 2(g) → CO 2(g) + heat 2 H 2 + O 2 → 2 H 2 O(g) + heat • Why?
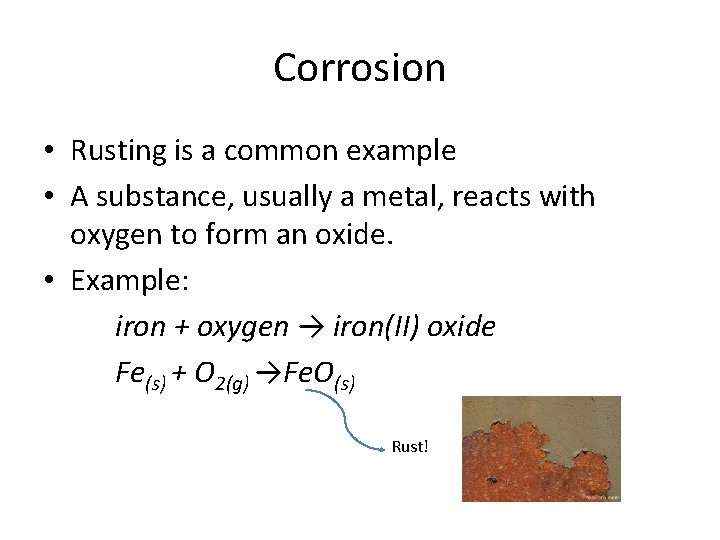
Corrosion • Rusting is a common example • A substance, usually a metal, reacts with oxygen to form an oxide. • Example: iron + oxygen → iron(II) oxide Fe(s) + O 2(g) →Fe. O(s) Rust!

Corrosion • Another famous example of corrosion: Copper reacts with oxygen in the air and slowly becomes copper oxide which is green in colour!
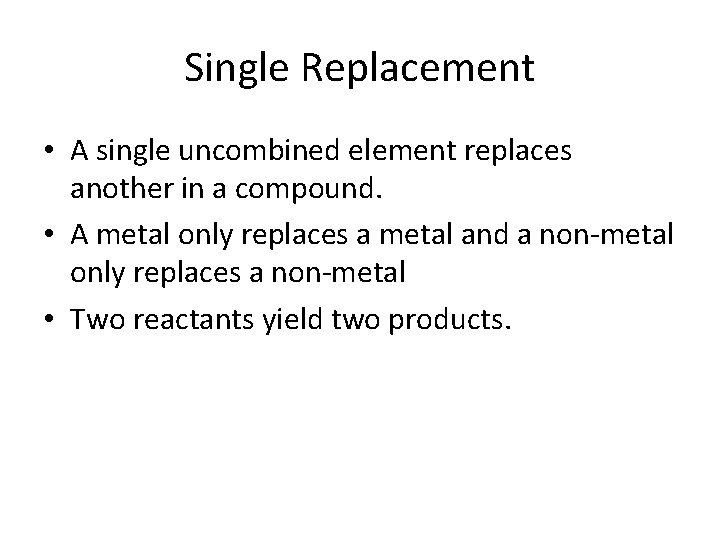
Single Replacement • A single uncombined element replaces another in a compound. • A metal only replaces a metal and a non-metal only replaces a non-metal • Two reactants yield two products.
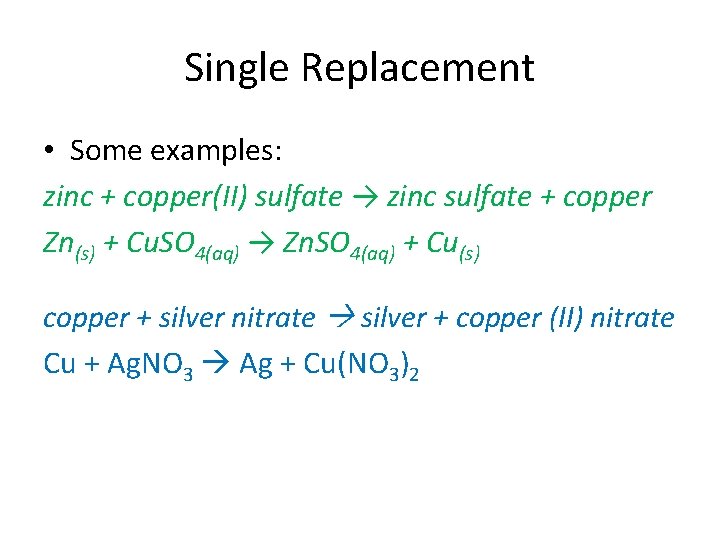
Single Replacement • Some examples: zinc + copper(II) sulfate → zinc sulfate + copper Zn(s) + Cu. SO 4(aq) → Zn. SO 4(aq) + Cu(s) copper + silver nitrate silver + copper (II) nitrate Cu + Ag. NO 3 Ag + Cu(NO 3)2
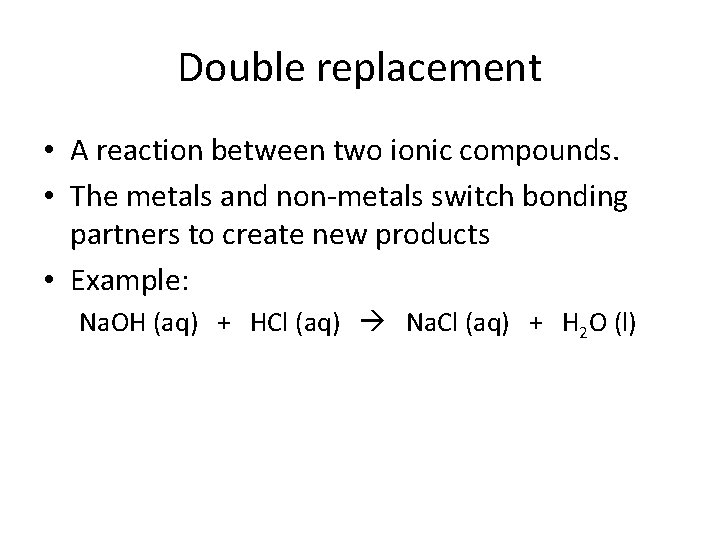
Double replacement • A reaction between two ionic compounds. • The metals and non-metals switch bonding partners to create new products • Example: Na. OH (aq) + HCl (aq) Na. Cl (aq) + H 2 O (l)

Chemical Bonds • When two atoms combine, the form a chemical bond. • Some bonds are weak (eg. Molecular compounds) • Some bonds are strong (eg. Ionic compounds) • To break bonds, we need to add energy. • When these bonds are broken, they release energy.
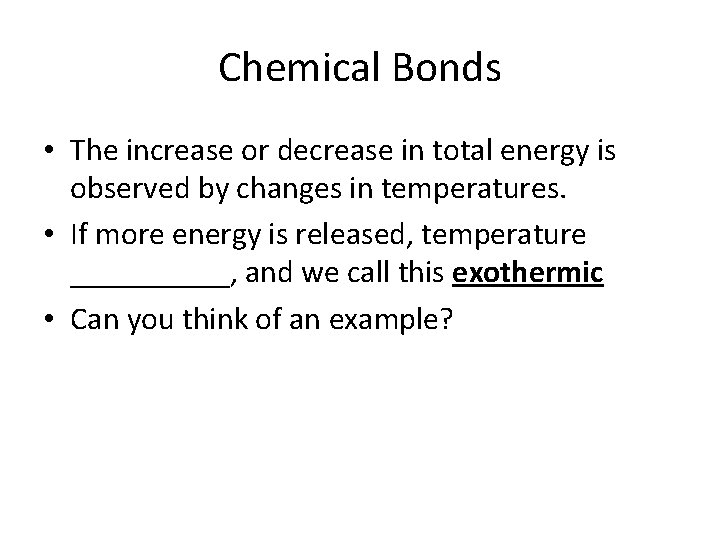
Chemical Bonds • The increase or decrease in total energy is observed by changes in temperatures. • If more energy is released, temperature _____, and we call this exothermic • Can you think of an example?
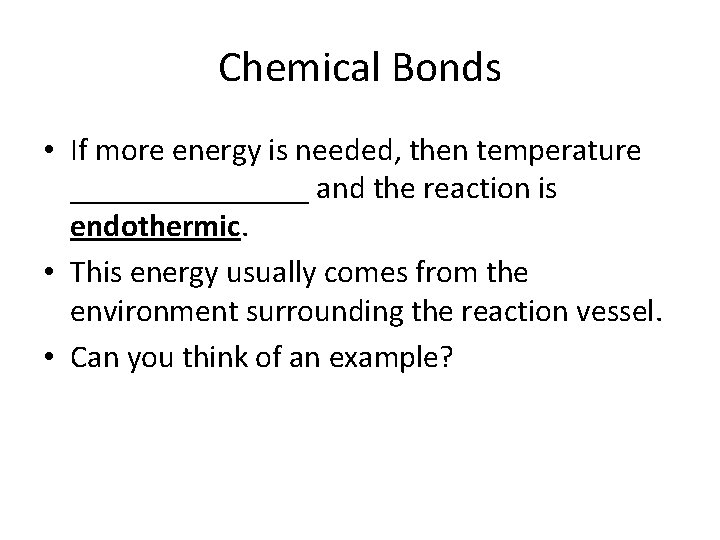
Chemical Bonds • If more energy is needed, then temperature ________ and the reaction is endothermic. • This energy usually comes from the environment surrounding the reaction vessel. • Can you think of an example?
- Slides: 16