Chemical Reactions Chemistry Joke Oxygen I went out















- Slides: 21

Chemical Reactions

Chemistry Joke Oxygen: I went out with potassium last night! How’d it go? OK!!

Introductio n v. Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken. v. Chemical reactions involve changes in matter—the making of new materials with new properties accompanied by energy changes. v. Chemical Reactions are described using shorthand called chemical

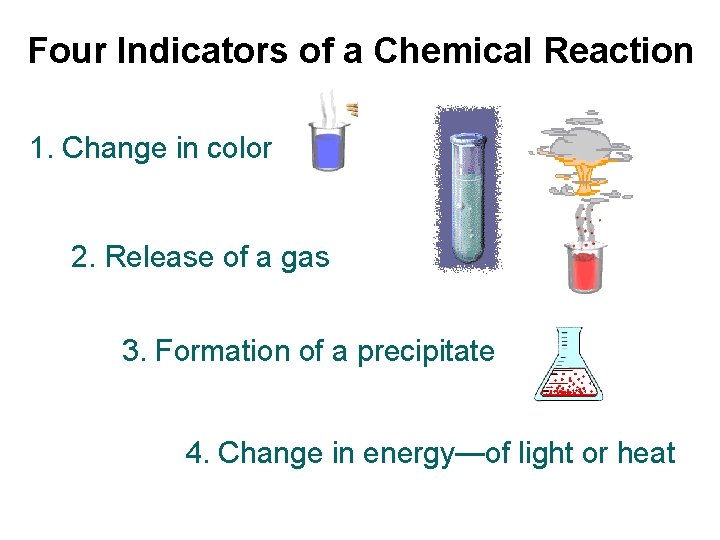
Four Indicators of a Chemical Reaction 1. Change in color 2. Release of a gas 3. Formation of a precipitate 4. Change in energy—of light or heat

But… The only absolute proof of a chemical reaction is that a new substance has been produced!

Chemical Equations A+B C+D REACTANTS Left of Arrow: Substances that existed before the reaction took PRODUCTS Right of Arrow: Substances that are produced in the reaction.

Three Types of Equations… ¢ 1. ) Word Equations l l ¢ 2. ) Formula Equations l l ¢ Propane + Oxygen Carbon Dioxide + Water + Energy Can see the products and the reactants C 3 H 8 + O 2 CO 2 + H 2 O + energy Can see the formulas & how many atoms of each element are reacting 3. ) Formula Equations w/ physical state indicated l l H 2(g) + O 2(g) H 2 O(l) + energy Indicates state of matter in reaction

Symbols Used in a Chemical Equation , yields , reacts with Mn. O 2 catalyst was used (catalyst written above the arrow)

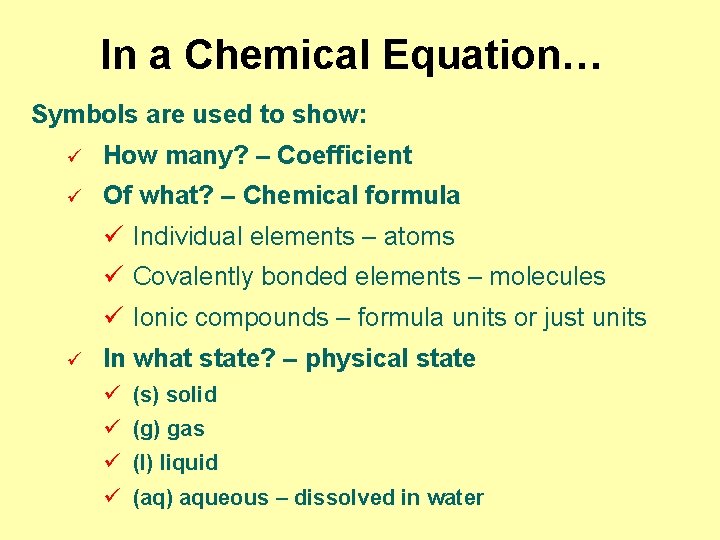
In a Chemical Equation… Symbols are used to show: ü How many? – Coefficient ü Of what? – Chemical formula ü Individual elements – atoms ü Covalently bonded elements – molecules ü Ionic compounds – formula units or just units ü In what state? – physical state ü (s) solid ü (g) gas ü (l) liquid ü (aq) aqueous – dissolved in water

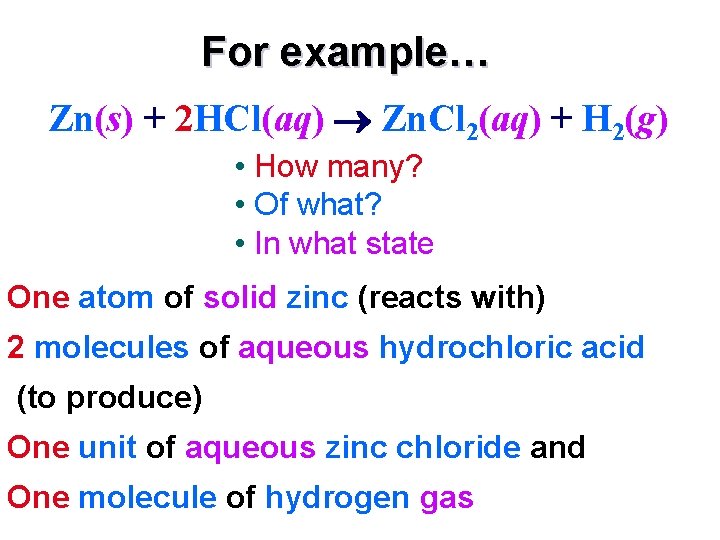
For example… Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) • How many? • Of what? • In what state? One atom of solid zinc (reacts with) 2 molecules of aqueous hydrochloric acid (to produce) One unit of aqueous zinc chloride and One molecule of hydrogen gas

Try one backwards… One molecule of oxygen gas ¢ Reacts with ¢ Two molecules of hydrogen gas ¢ To produce ¢ Two molecules of liquid water. ¢ Write the equation. O 2 (g) + 2 H 2 (g) → 2 H 2 O (l)

Try another one… Two atoms of aluminum react with three units of aqueous copper (II) chloride to produce three atoms of copper and two units of aqueous aluminum chloride. • How many? • Of what? • In what state? 2 Al(s) + 3 Cu. Cl 2(aq) 3 Cu(s) + 2 Al. Cl 3(aq)

In a Chemical Reaction… Mass is neither created nor destroyed—Law of the Conservation of Mass and Energy ü ü Total mass stays the same Atoms are only rearranged, not changed into different ones 4 H 4 H 36 g 2 O 4 g 32 g 2 O

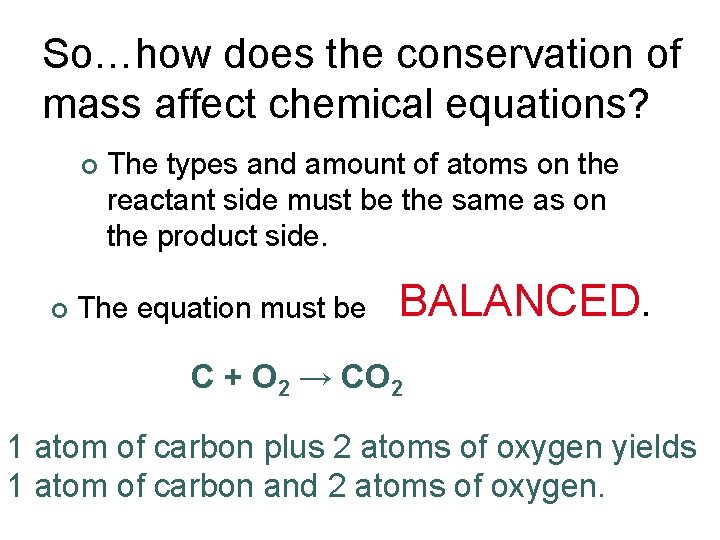
So…how does the conservation of mass affect chemical equations? ¢ ¢ The types and amount of atoms on the reactant side must be the same as on the product side. The equation must be BALANCED. C + O 2 → CO 2 1 atom of carbon plus 2 atoms of oxygen yields 1 atom of carbon and 2 atoms of oxygen.

Balancing Equations 1. Write the unbalanced equation. 2. Count atoms on each side. 3. Add coefficients to make #s equal. Coefficient subscript = # atoms 4. Reduce coefficients to lowest possible ratio. 5. Double check atom balance!!!

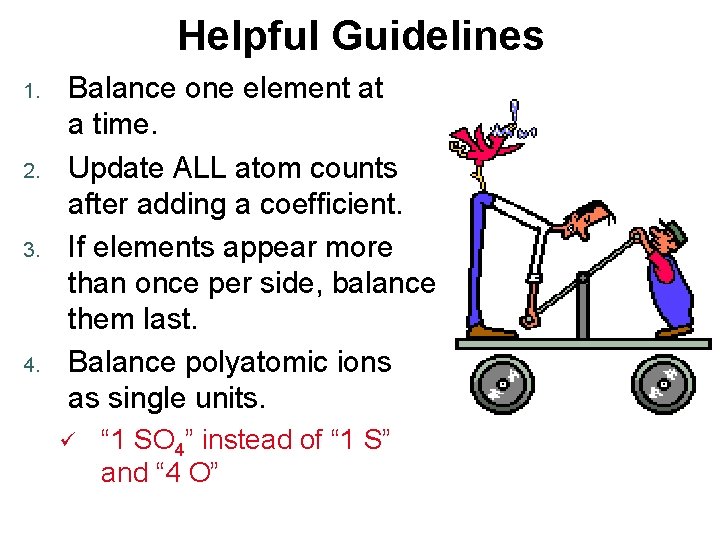
Helpful Guidelines 1. 2. 3. 4. Balance one element at a time. Update ALL atom counts after adding a coefficient. If elements appear more than once per side, balance them last. Balance polyatomic ions as single units. ü “ 1 SO 4” instead of “ 1 S” and “ 4 O”

More Helpful Guidelines 5. 6. Balance diatomic molecules last. Remember: change coefficients only—not subscripts. Go in this order: Metals Nonmetals except H & O Polyatomics H O

Balancing Example Aluminum and copper(II)chloride form copper and aluminum chloride. 2 Al + 3 Cu. Cl 2 3 Cu + 2 Al. Cl 3 21 / Al 1/ 2 3 1/ Cu 1/ 3 6 /2 Cl 3/ 6

Try these examples… ¢ C 2 H 4 + O 2 CO 2 + H 2 O l ¢ Ag. NO 3 + Cu Cu(NO 3)2 + Ag l ¢ C 2 H 4 + 3 O 2 2 CO 2 + 2 H 2 O 2 Ag. NO 3 + Cu (NO 3)2 + 2 Ag Fe(OH)3 Fe 2 O 3 + H 2 O l 2 Fe(OH)3 Fe 2 O 3 + 3 H 2 O

Try these examples… ¢ NH 3 + O 2 NO + H 2 O l ¢ 4 NH 3 + 5 O 2 4 NO + 6 H 2 O NH 3 + O 2 NO 2 + H 2 O l 4 NH 3 + 7 O 2 4 NO 2 + 6 H 2 O

Chemistry Joke Sick Chemistry Humor From way down in my cranium This prediction I will make: … That if you eat uranium You’ll get atomic ache!