Chemical Reactions Chemical Reaction Two or more substances

Chemical Reactions
Chemical Reaction • Two or more substances combine to form a new substance. Indicators (indicate a reaction has taken place) – – energy: heat, light, cold color change production of gas odor
Formula for Chemical Reactions • reactant + reactant = product • Endothermic reaction: energy is required; heat absorbed; cold • Exothermic reaction: energy is given off; heat, light • Catalyst: substance that speeds up the reaction (but remains unchanged) • Inhibitor: substance that prevents reaction from taking place

Types of Reactions We classify reactions into categories so that we may more easily predict the products.
Synthesis Reaction Synthesis reaction – 2 or more substances combine to form a more complex substance. A + B AB +

Synthesis Examples 2 Mg + O 2 2 Mg. O 2 H 2 + O 2 2 H 2 O
Decomposition Reaction Decomposition reaction – Opposite of synthesis reaction. A more complex substance is broken down into a less complex substance. AB A + B +
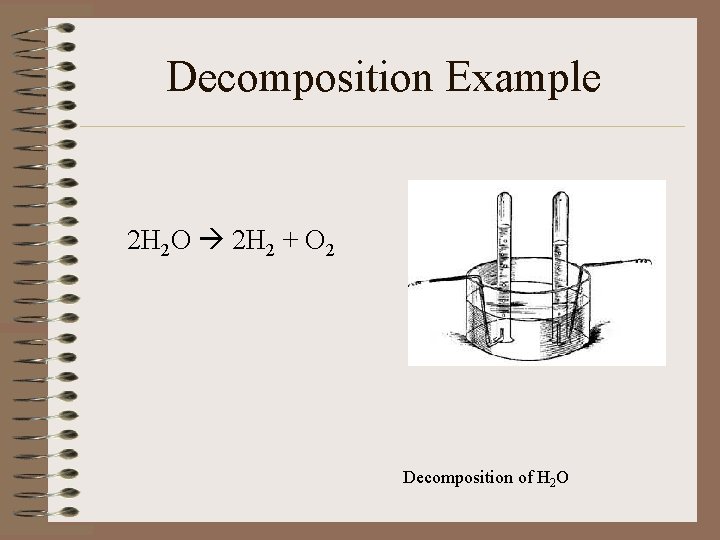
Decomposition Example 2 H 2 O 2 H 2 + O 2 Decomposition of H 2 O
Single Displacement Reaction Single Displacement reaction – When a metal or non-metal replaces a metal or nonmetal in a more complex substance. A + BY AY + B + +
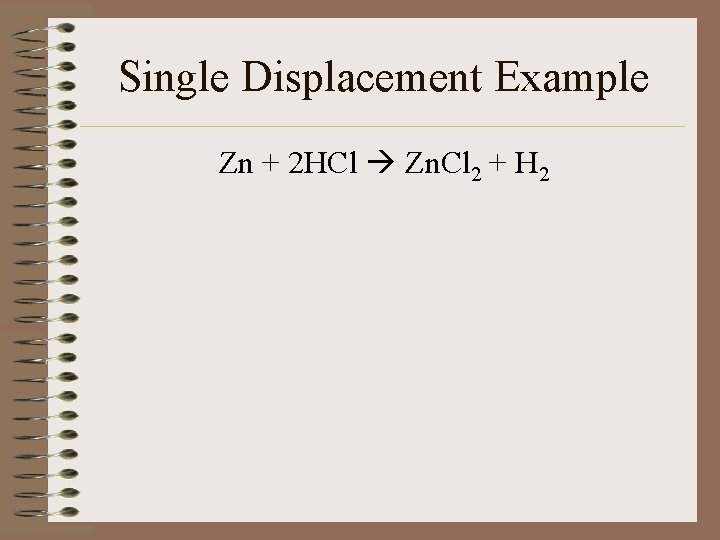
Single Displacement Example Zn + 2 HCl Zn. Cl 2 + H 2
Double Displacement reaction – When two metal or non-metals in separate complex substances replace each other to form two new complex substances. AX + BY AY + BX + +

Double Displacement Examples Mg. O + Be. S Mg. S + Be. O Na 2 S + Zn(NO 3)2 2 Na(NO 3) + Zn. S
- Slides: 13