Chemical Reactions Chemical Bonds Covalent Bonds Sharing of
Chemical Reactions

Chemical Bonds

Covalent Bonds • Sharing of a pair of valence electrons by two atoms.
Covalent Bonds • Non-polar covalent bond: – Occurs when the two atoms are equally electronegative (EN). – Electrons shared equally. • Polar covalent bond: – Occurs when one atom is more EN than the other. – Electrons are not shared equally.
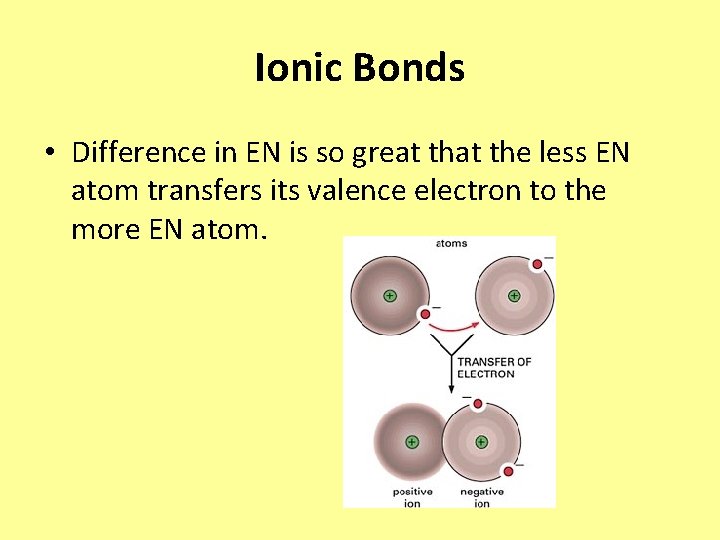
Ionic Bonds • Difference in EN is so great the less EN atom transfers its valence electron to the more EN atom.
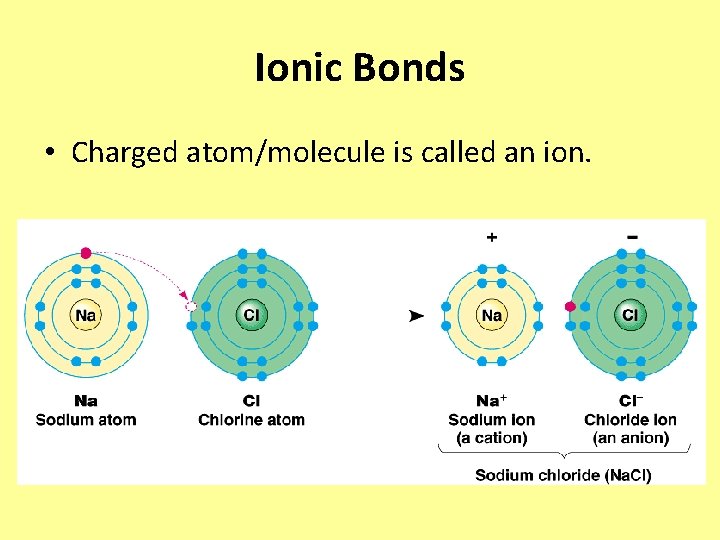
Ionic Bonds • Charged atom/molecule is called an ion.

Anabolic Reactions (building up)
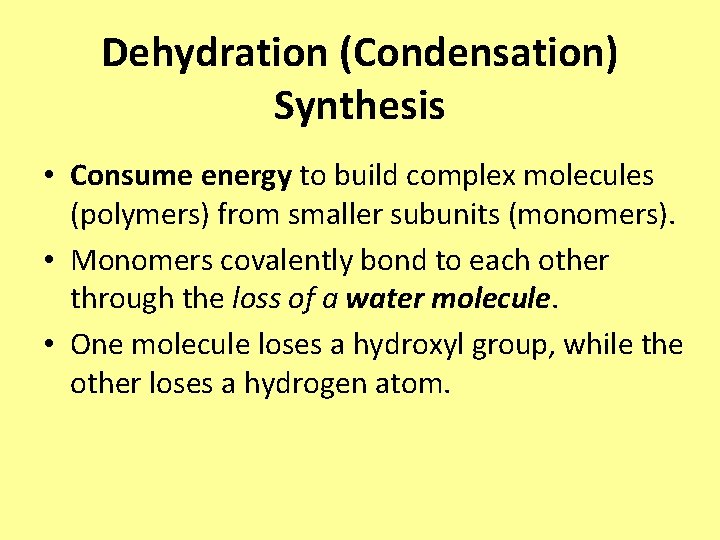
Dehydration (Condensation) Synthesis • Consume energy to build complex molecules (polymers) from smaller subunits (monomers). • Monomers covalently bond to each other through the loss of a water molecule. • One molecule loses a hydroxyl group, while the other loses a hydrogen atom.
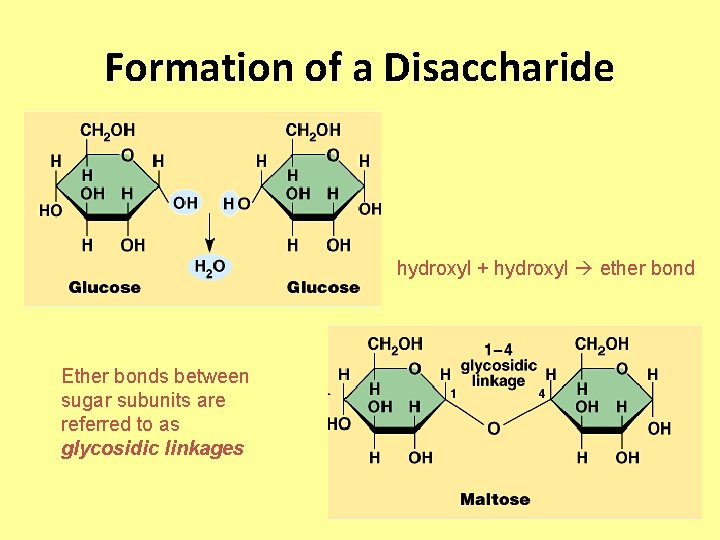
Formation of a Disaccharide hydroxyl + hydroxyl ether bond Ether bonds between sugar subunits are referred to as glycosidic linkages

Formation of a Triglyceride hydroxyl + carboxyl ester bond Linkage between glycerol and fatty acids are ester linkages
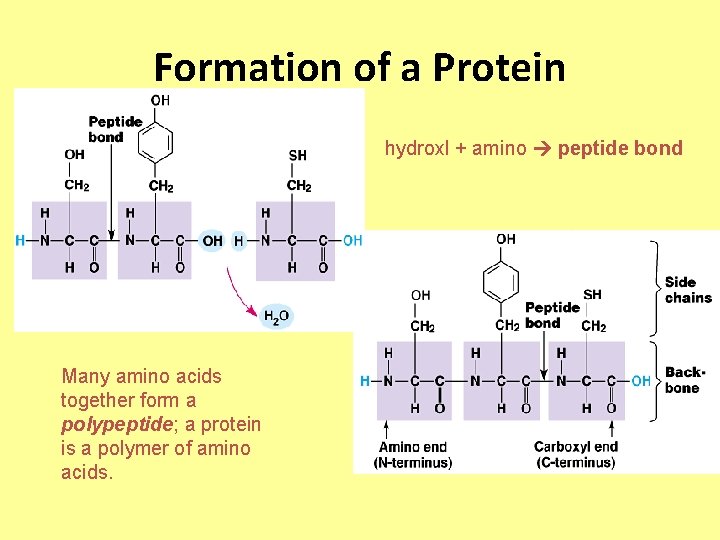
Formation of a Protein hydroxl + amino peptide bond Many amino acids together form a polypeptide; a protein is a polymer of amino acids.
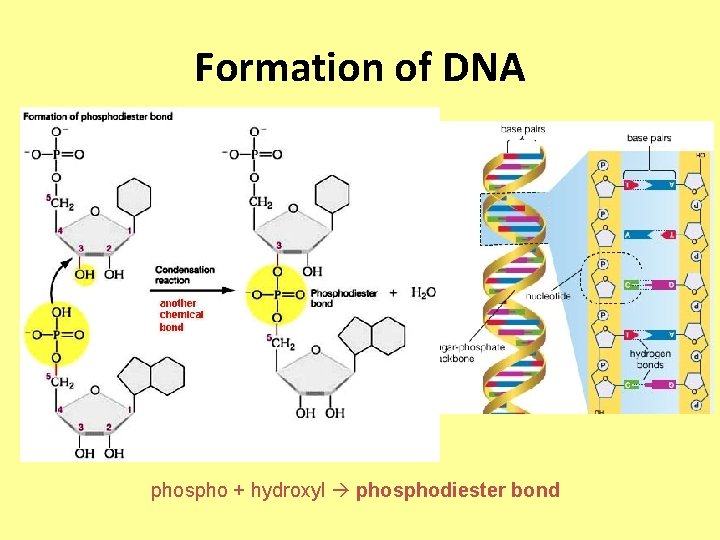
Formation of DNA phospho + hydroxyl phosphodiester bond

Catabolic Reactions (breaking down)
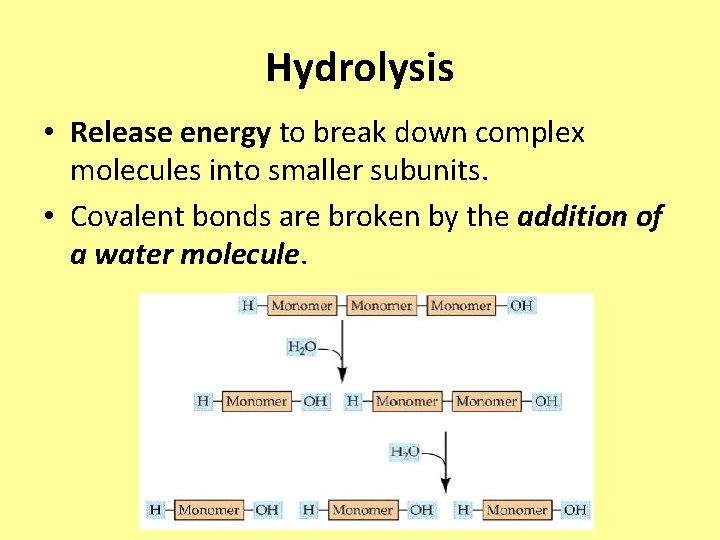
Hydrolysis • Release energy to break down complex molecules into smaller subunits. • Covalent bonds are broken by the addition of a water molecule.

Oxidation-Reduction (Redox) Reactions

Redox Reactions • Releases energy as one or more electrons released from one substance move closer to EN atoms. • Oxidation: – Loss of electrons from one substance. • Reduction: – Addition of electrons to another substance.
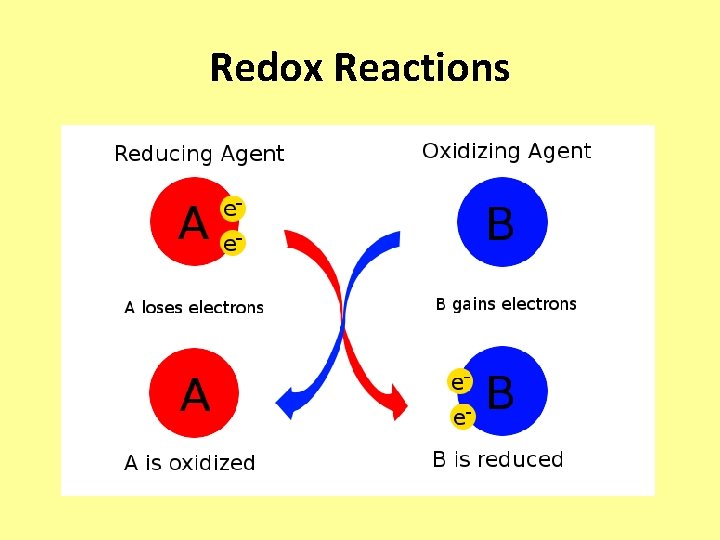
Redox Reactions
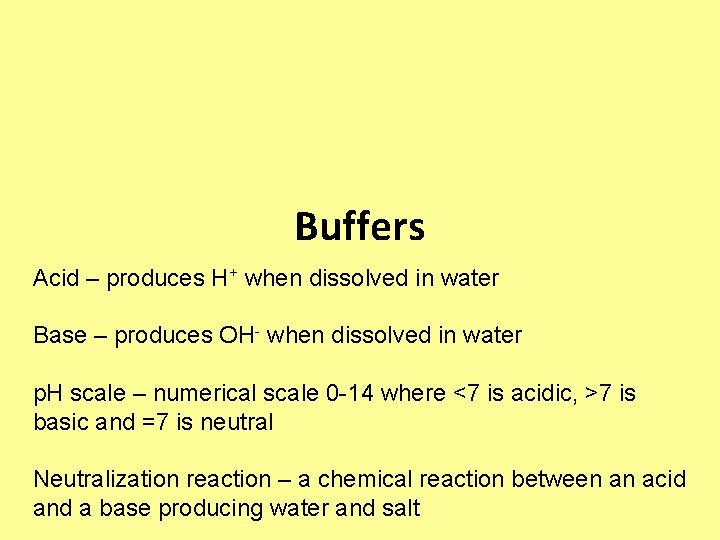
Buffers Acid – produces H+ when dissolved in water Base – produces OH- when dissolved in water p. H scale – numerical scale 0 -14 where <7 is acidic, >7 is basic and =7 is neutral Neutralization reaction – a chemical reaction between an acid and a base producing water and salt
![Buffers • Substances that minimize changes in the [H+] and [OH-] in a solution. Buffers • Substances that minimize changes in the [H+] and [OH-] in a solution.](http://slidetodoc.com/presentation_image_h2/0c23cf23c84bde68fb854e4dd3d5fb1c/image-19.jpg)
Buffers • Substances that minimize changes in the [H+] and [OH-] in a solution. • Chemical Equilibrium between carbonic acid and bicarbonate acts as a p. H regulator.
- Slides: 19