Chemical Reactions Chapter 6 Counting Atoms Subscripts indicate

Chemical Reactions Chapter 6

Counting Atoms Subscripts indicate the number of atoms in a COMPOUND

Example H 2 O 2 Hydrogen Atoms 1 Oxygen The number 1 is INVISIBLE
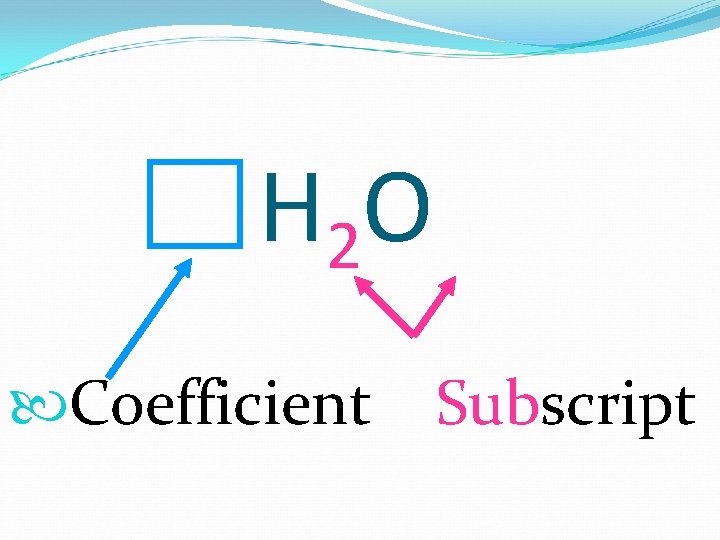
H 2 O Coefficient Subscript
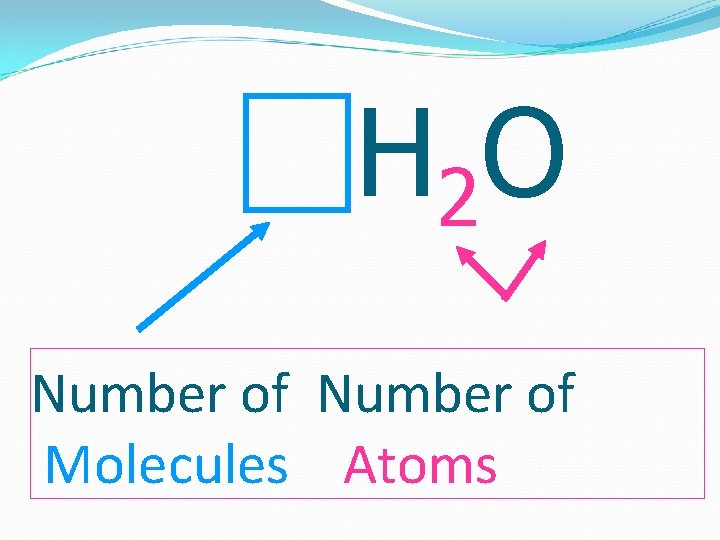
H 2 O Number of Molecules Atoms

2 Ag 1 Cl 2 MULTIPLY Coefficient x Subscript
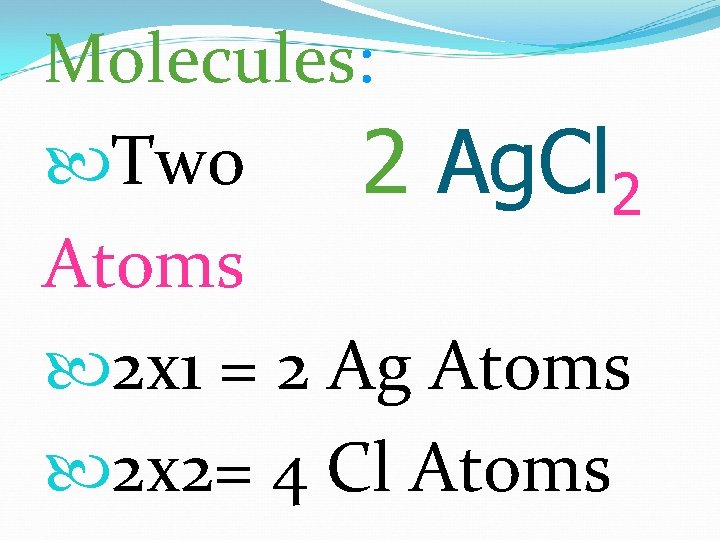
Molecules: Two 2 Ag. Cl 2 Atoms 2 x 1 = 2 Ag Atoms 2 x 2= 4 Cl Atoms

4 Na. Cl Molecules = 4 Atoms: 4 Sodium Atoms 4 Chlorine Atoms

2 Ca(OH)2 Molecules = 2 Atoms: 2 Calcium Atoms 4 Oxygen Atoms 4 Hydrogen

3 H 2 O Molecules = 3 Atoms: 6 Hydrogen Atoms 3 Oxygen Atoms

4 CO 2 Molecules = 4 Atoms: 4 Carbon Atoms 8 Oxygen Atoms

Chemical Equations
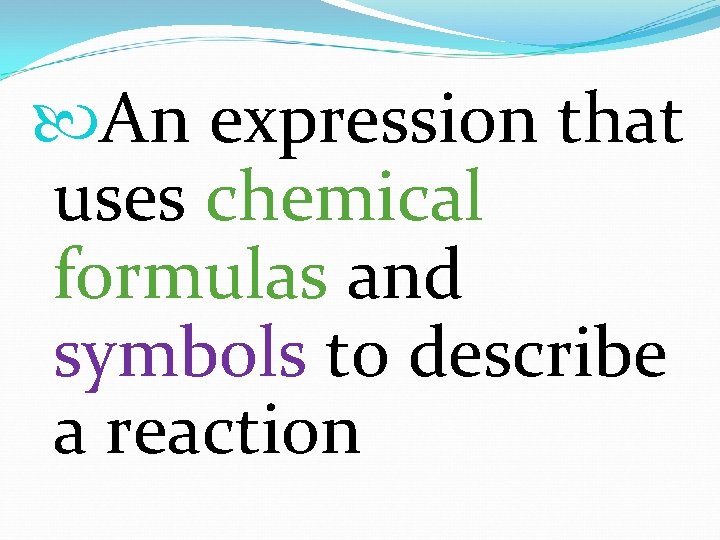
An expression that uses chemical formulas and symbols to describe a reaction
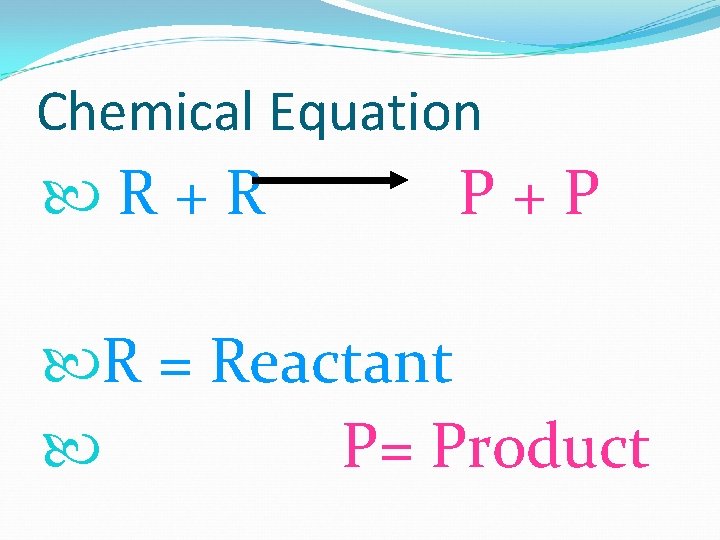
Chemical Equation R+R P+P R = Reactant P= Product

Reactants Start the reaction At the Beginning LEFT SIDE OF ARROW

“ARROW” Yields

Products New substance(s) Product = Finished RIGHT OF THE ARROW

Identify Products and Reactants
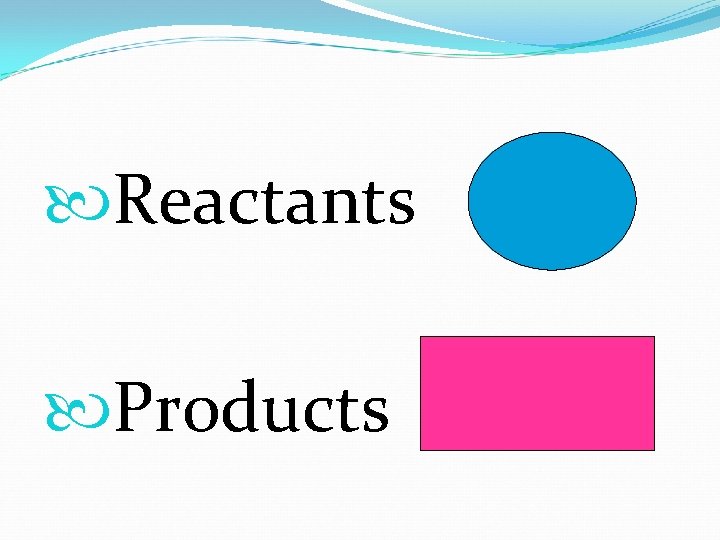
Reactants Products
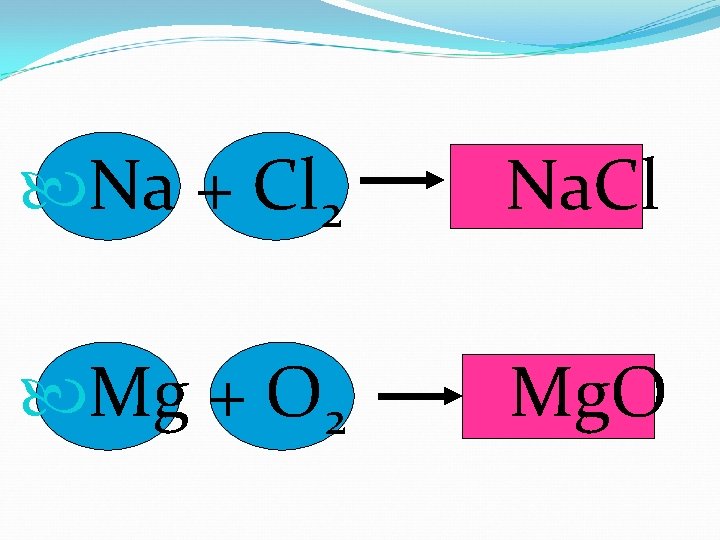
Na + Cl 2 Na. Cl Mg + O 2 Mg. O
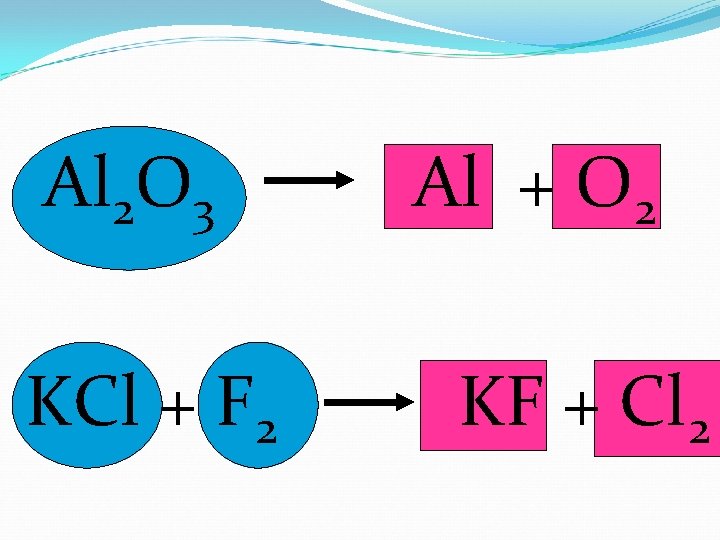
Al 2 O 3 KCl + F 2 Al + O 2 KF + Cl 2

Why Balance Equations?

To obey the Law of Conservation of Mass

Atoms CANNOT be created or destroyed Mass of reactants = Mass of products
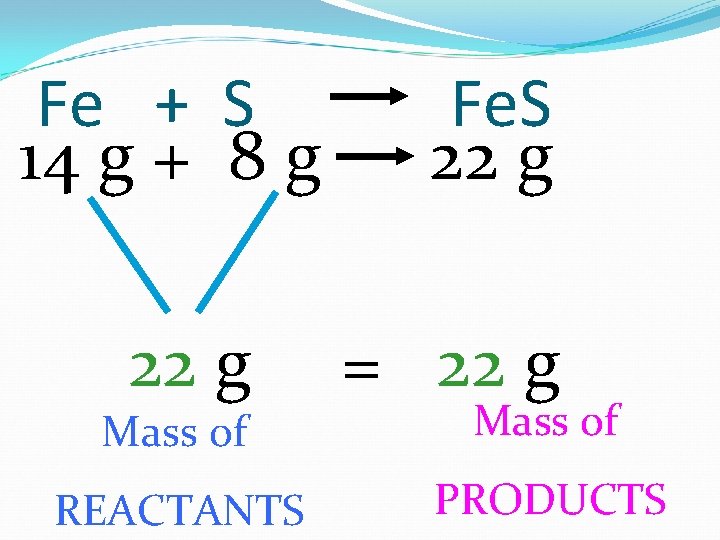
Fe + S 14 g + 8 g 22 g Mass of REACTANTS Fe. S 22 g = 22 g Mass of PRODUCTS
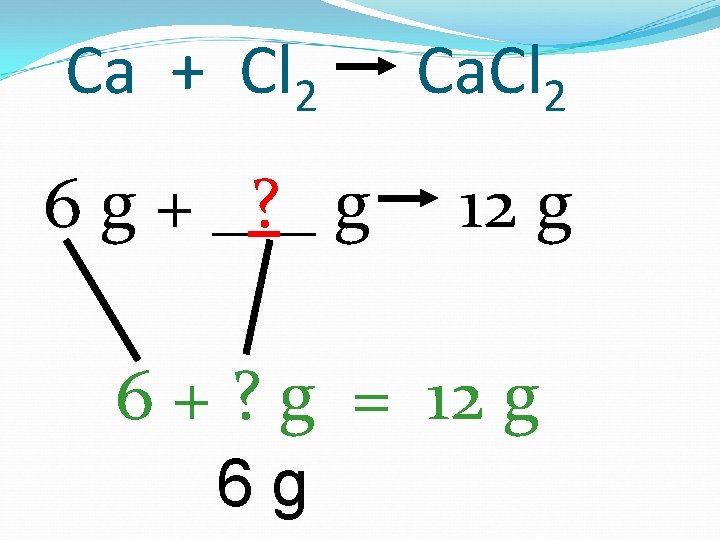
Ca + Cl 2 6 g + _? _ g Ca. Cl 2 12 g 6 + ? g = 12 g 6 g

Fe + S ? g + 16. 0 g Fe. S 44. 0 g ? + 16. 0 g = 44. 0 g 28. 0 g
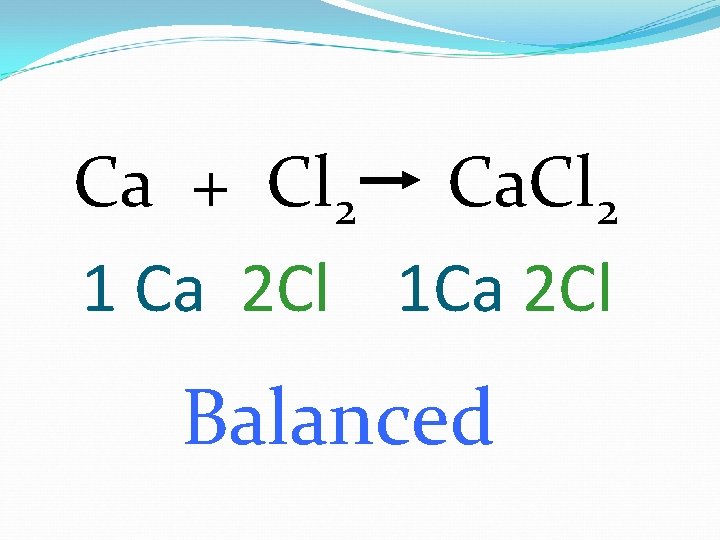
Ca + Cl 2 Ca. Cl 2 1 Ca 2 Cl 1 Ca 2 Cl Balanced
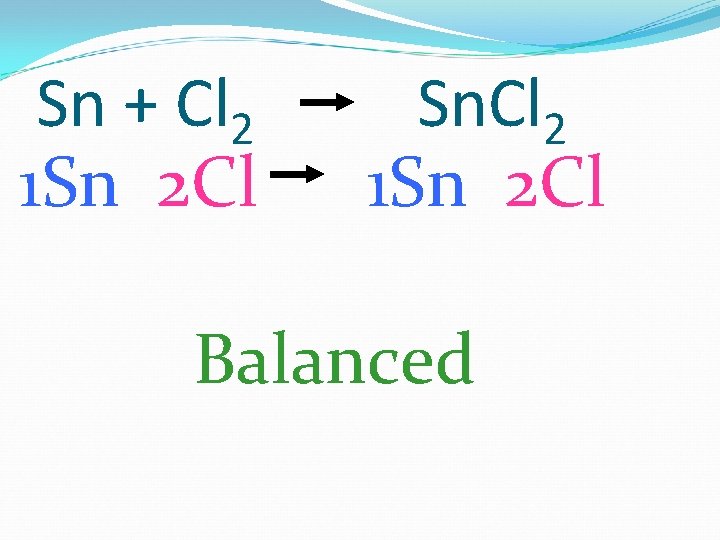
Sn + Cl 2 1 Sn 2 Cl Sn. Cl 2 1 Sn 2 Cl Balanced
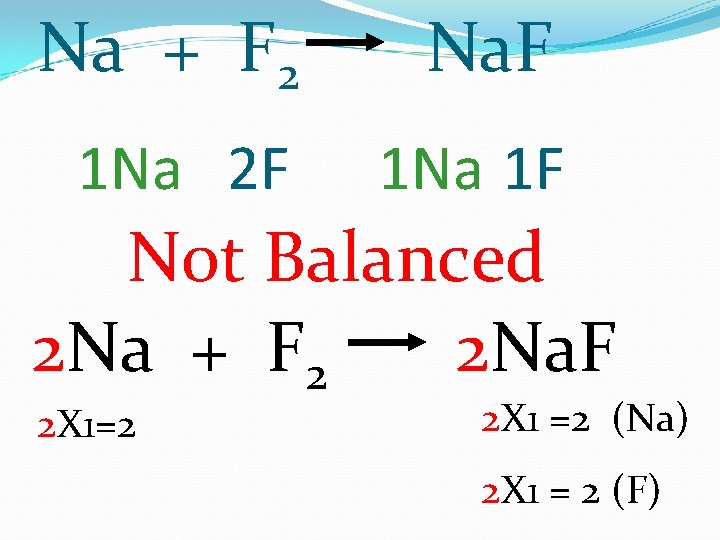
Na + F 2 Na. F 1 Na 2 F 1 Na 1 F Not Balanced 2 Na + F 2 2 Na. F 2 X 1=2 2 X 1 =2 (Na) 2 X 1 = 2 (F)

Balancing Chemical Equations Step 1: Write the equation

Step 2: Count the Atoms

Step 3: Use COEFFICIENTS to Balance Atoms
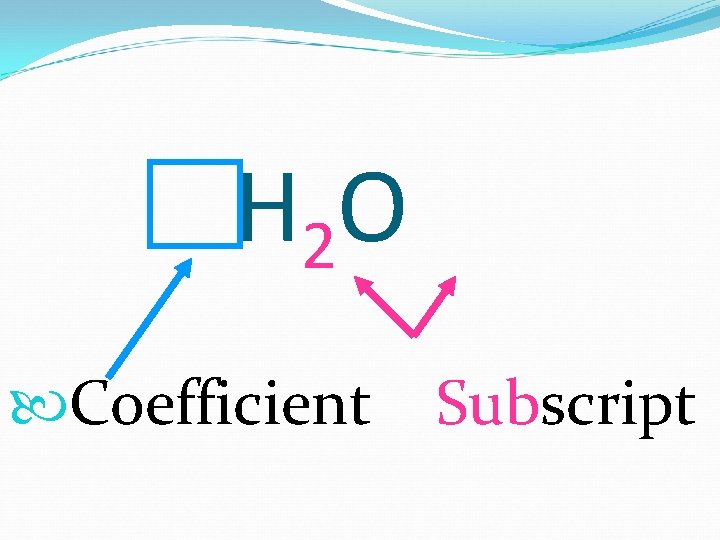
H 2 O Coefficient Subscript

Step 4: Double Check Math

Balancing Reactions
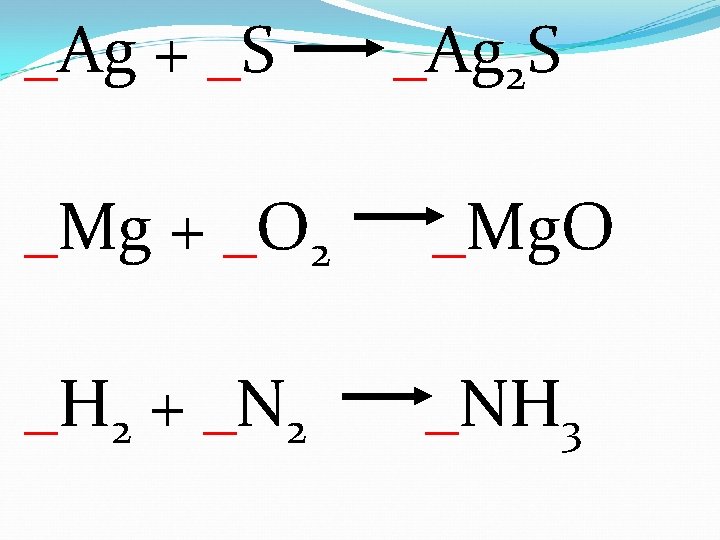
_Ag + _S _Ag 2 S _Mg + _O 2 _Mg. O _H 2 + _N 2 _NH 3
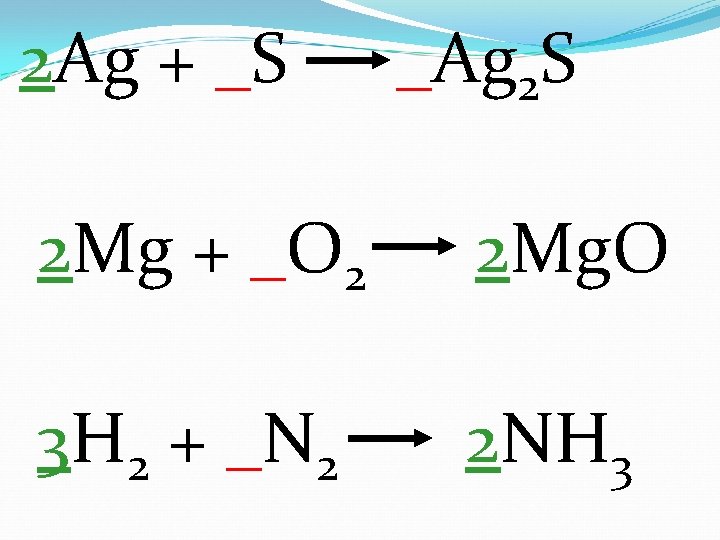
2 Ag + _S _Ag 2 S 2 Mg + _O 2 2 Mg. O 3 H 2 + _N 2 2 NH 3

Classifying Chemical Reactions
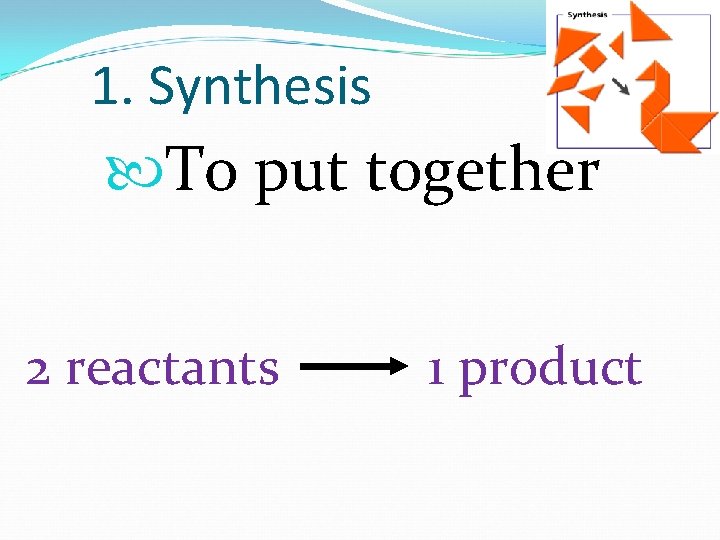
1. Synthesis To put together 2 reactants 1 product
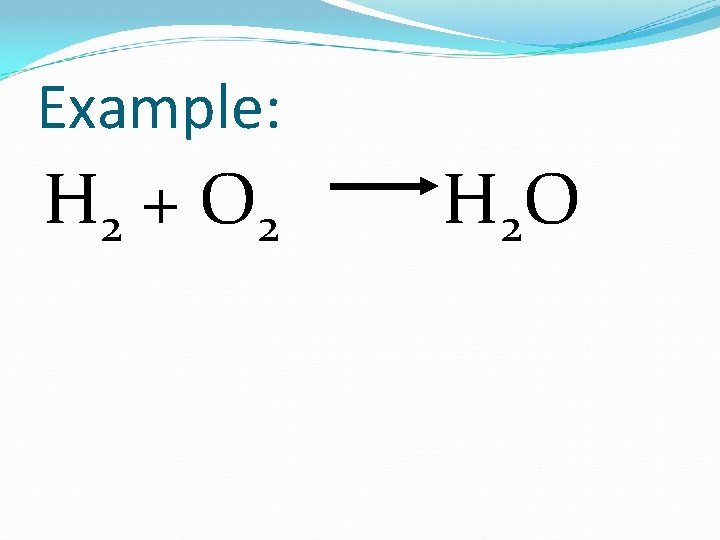
Example: H 2 + O 2 H 2 O

2. Decomposition To tear apart (breakdown) 1 reactant 2 products
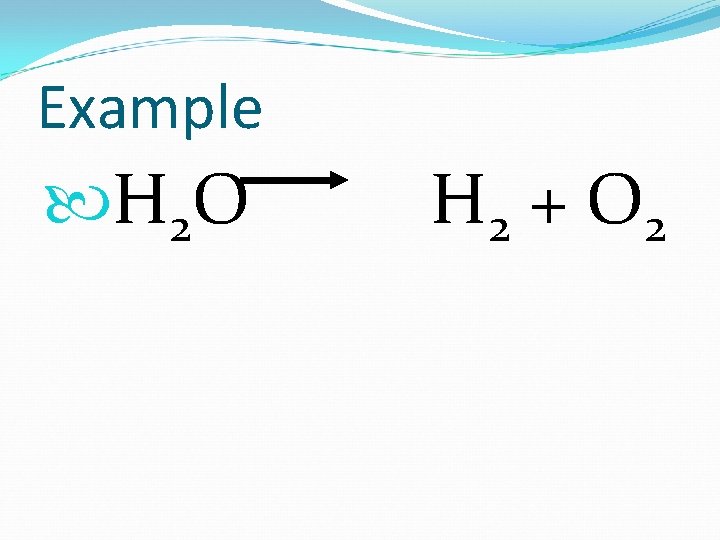
Example H 2 O H 2 + O 2

3. Single Replacement 2 reactants 2 products Look for a SINGLE element!
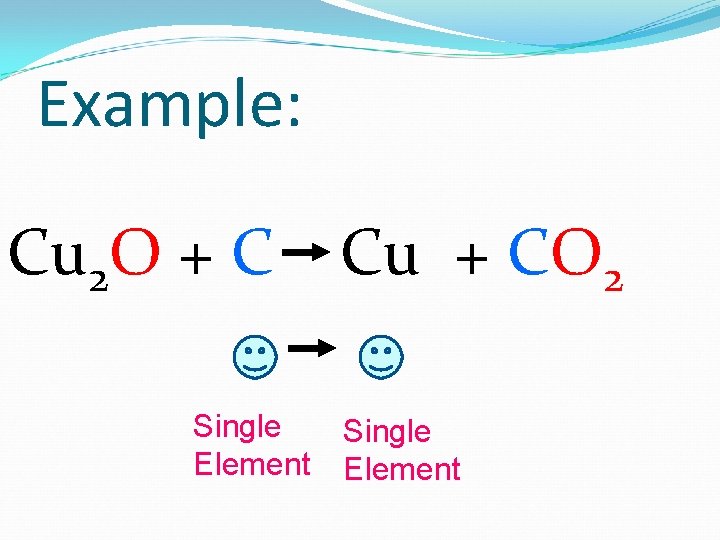
Example: Cu 2 O + C Single Element Cu + CO 2 Single Element
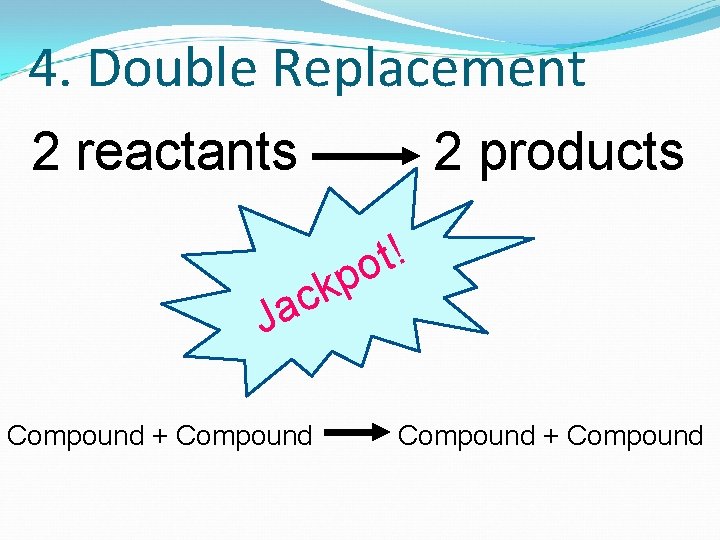
4. Double Replacement 2 reactants 2 products ! t o p ck Ja Compound + Compound
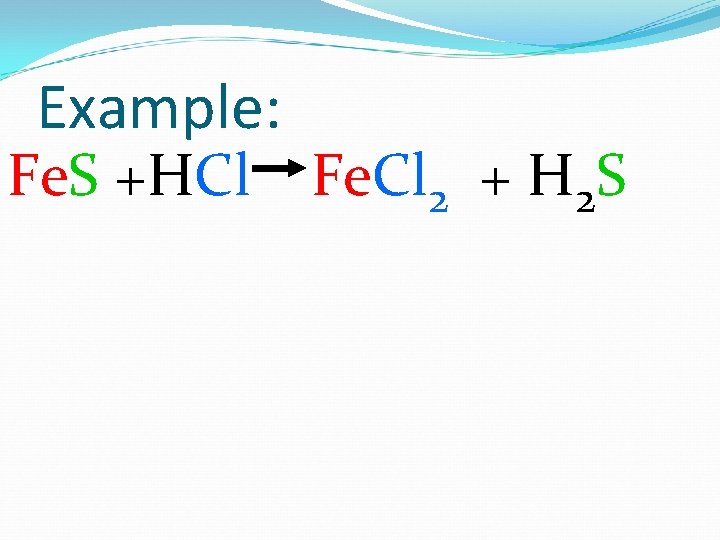
Example: Fe. S +HCl Fe. Cl 2 + H 2 S
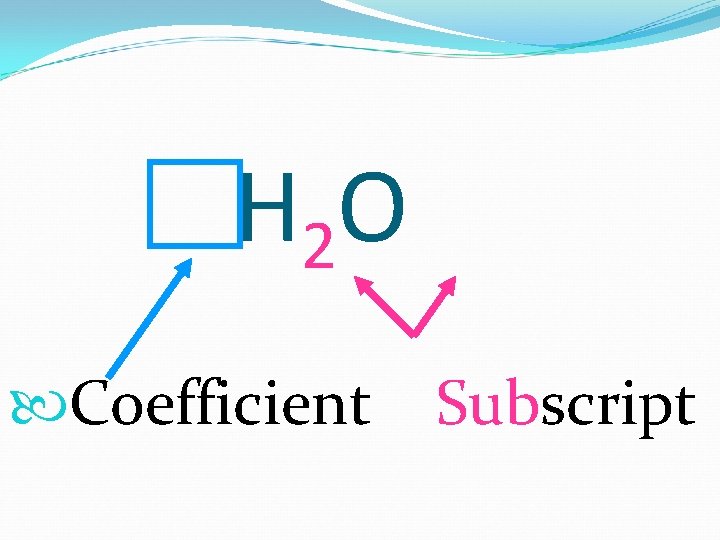
H 2 O Coefficient Subscript
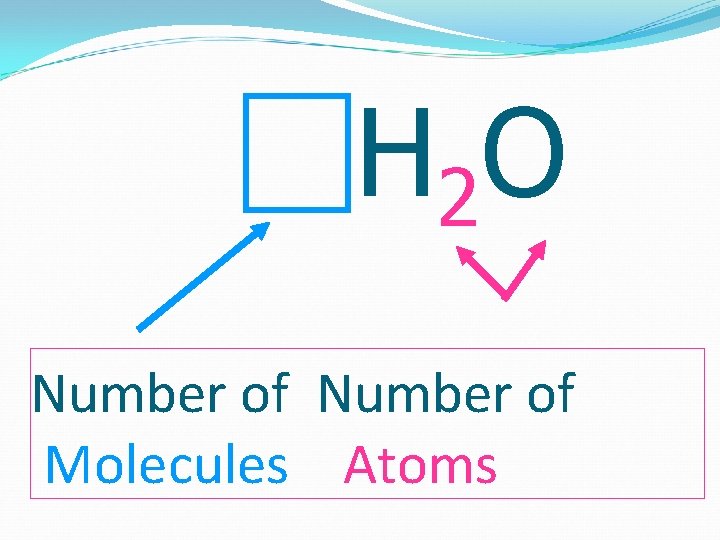
H 2 O Number of Molecules Atoms

2 Ag 1 Cl 2 MULTIPLY Coefficient x Subscript
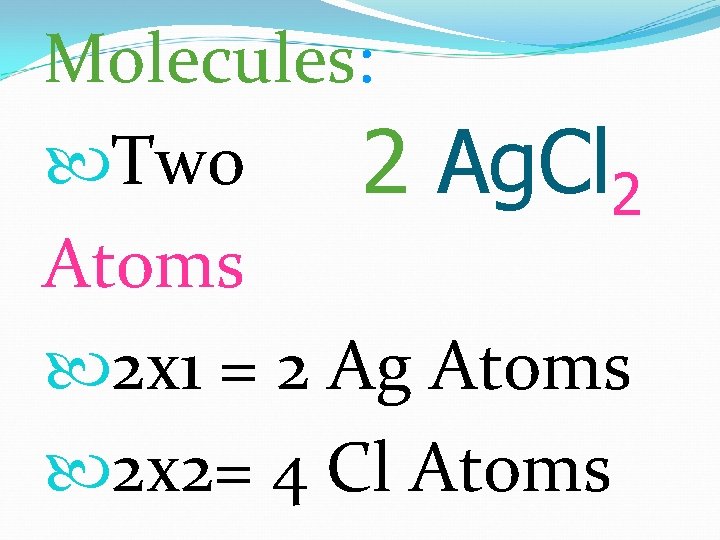
Molecules: Two 2 Ag. Cl 2 Atoms 2 x 1 = 2 Ag Atoms 2 x 2= 4 Cl Atoms

4 Na. Cl Molecules = 4 Atoms: 4 Sodium Atoms 4 Chlorine Atoms

3 H 2 O Molecules = 3 Atoms: 6 Hydrogen Atoms 3 Oxygen Atoms

4 CO 2 Molecules = 4 Atoms: 4 Carbon Atoms 8 Oxygen Atoms

Acids and Bases

Indicators Compounds that change color when it contacts an acid or a base

Properties of Acids Sour taste Reacts with metals (corrosive) Turns litmus paper red

Properties of Bases Bitter Taste Slippery Feel Turns Litmus paper Blue


Acids Produce Hydrogen + Ions (H ) in water

Bases Produce Hydroxide ions (OH ) in water

The p. H scale
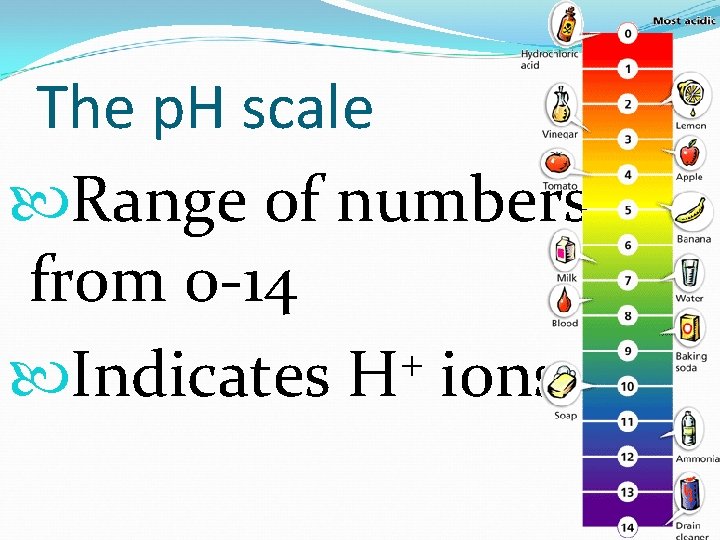
The p. H scale Range of numbers from 0 -14 + Indicates H ions
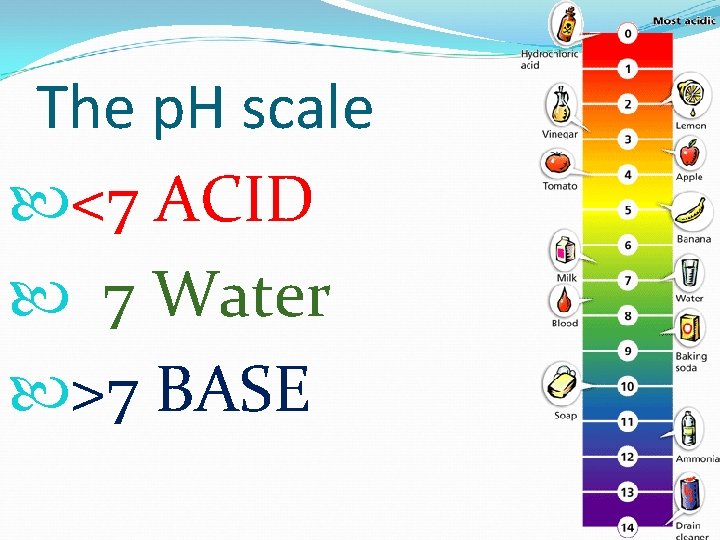
The p. H scale <7 ACID 7 Water >7 BASE

- Slides: 65