Chemical Reactions Chapter 2 States of Matter Before
Chemical Reactions Chapter 2
States of Matter • Before we can move on, you must be able to predict the states of matter for different substances HINTS: • For molecular substances – MEMORIZE!! • For solid, pure metals – SOLID (except Hg, and where stated) • For others, CHECK YOUR DATA BOOKLET’S SOLUBILITY TABLE

Solubility Table • A solubility table allows us to determine whether or not an ionic compound will be soluble (able to dissolve) or not in water. • we will use the subscript (aq) behind the chemical if it is soluble in water • the subscript (s) if it is not soluble or insoluble
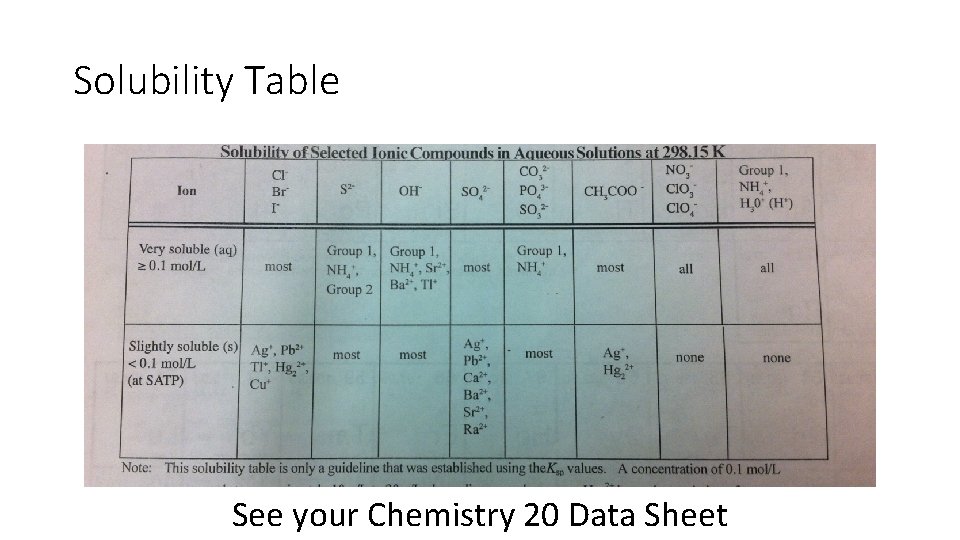
Solubility Table See your Chemistry 20 Data Sheet
How does the Solubility Table work? • Mostly anions along the top • The 2 nd row deals with high solubility • If you find the cation you need in this row, under the matching anion column, your compound will be aqueous (aq) – or soluble • The 3 rd row deals with low solubility • If you find the cation you need in this row, under the matching anion column, your compound will be solid (s) – or insoluble
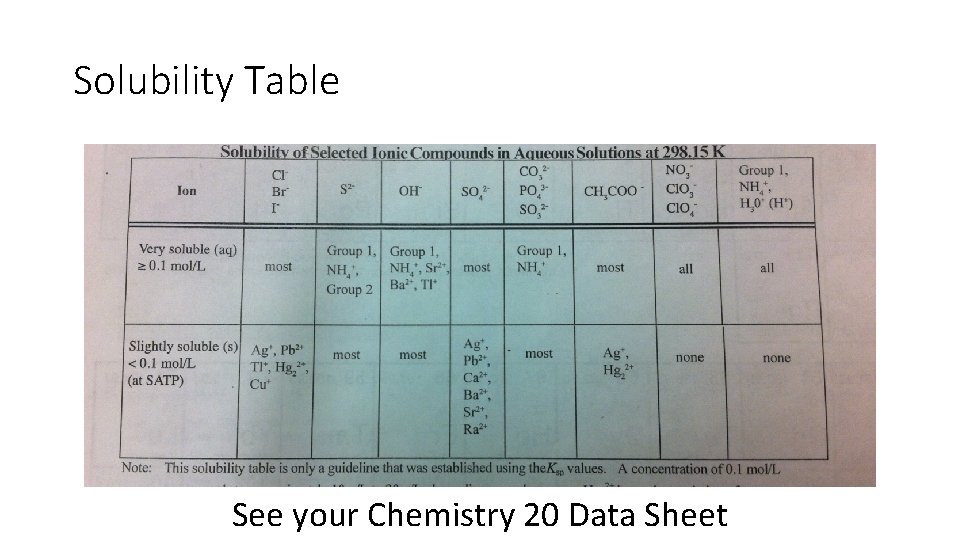
Solubility Table See your Chemistry 20 Data Sheet

You Try! • Na. OH • Ba(OH)2 • (NH 4)2 SO 4 • Ca(CH 3 COO)2

Hints / Rules of Thumb • All nitrate compounds are aqueous! • All ammonium compounds are aqueous! • Most metal oxides are solids
How and Why Chemical Reactions Occur • Based on the Kinetic Molecular Theory • Smallest entities (ions, atoms, or molecules) of a substance are in continuous motion • Because the particles are in constant motion, they collide with each other • When collisions have certain orientations and sufficient energy, components of the particles are rearranged to form new products

Chemical Reactions: • DO NOT cause atoms to be created or destroyed • Changes in energy are always associated with chemical reactions; this is due to the breaking and/or formation of chemical bonds. • Particles of the reactants must collide for reactions to occur. • Reactants must have a certain minimum energy for reactions to occur.
Evidence of a Chemical Reaction • New substance is produced • Energy is transferred • Possible observations: • • Colour change Odor change State change (gas bubbles, precipitate) Temperature change (endo/exothermic reactions)
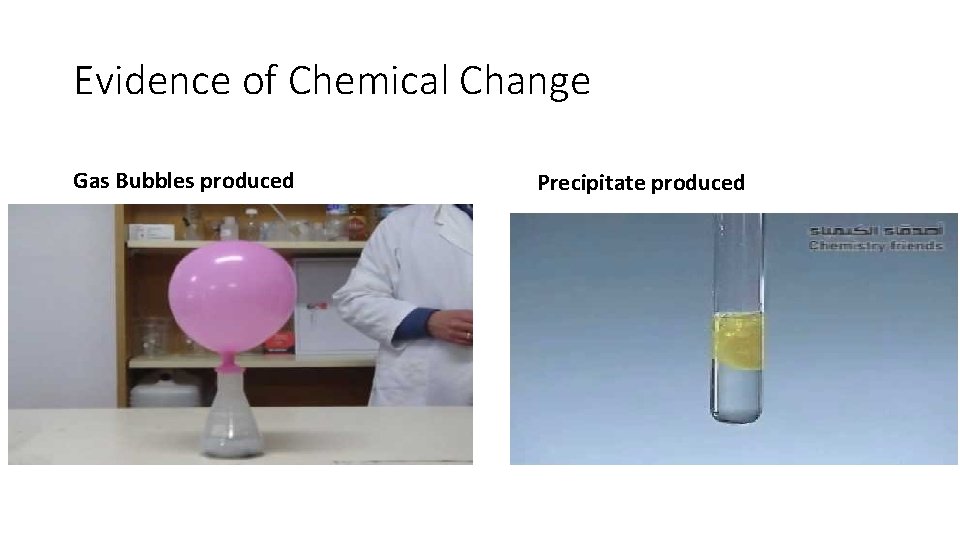
Evidence of Chemical Change Gas Bubbles produced Precipitate produced
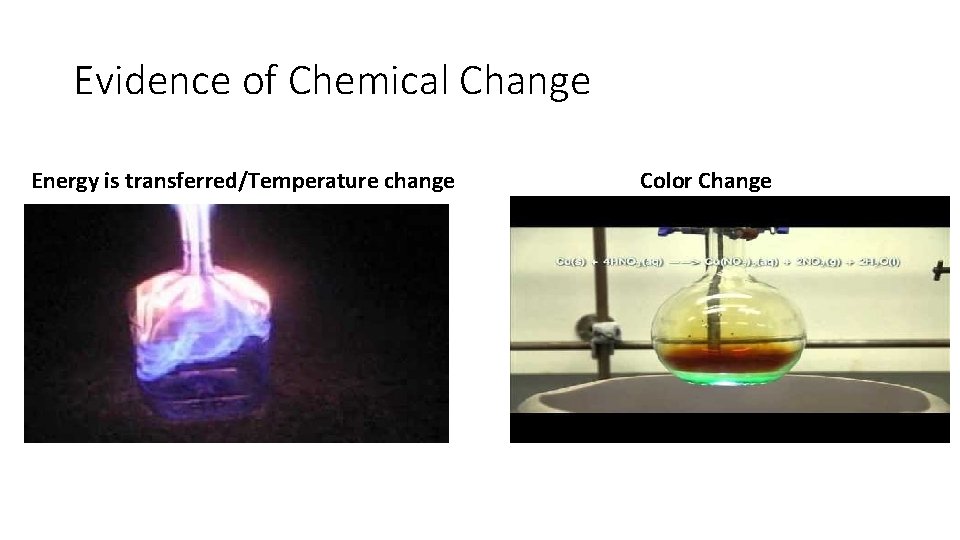
Evidence of Chemical Change Energy is transferred/Temperature change Color Change

Balancing Chemical Equations • A chemical reaction is like a recipe: • It tell us exactly how much of each substance is needed and how much is produced. • Chemical reactions involve the rearrangement of the reactant atoms in such a way so as to give us the products.
Balancing Chemical Equations • A balanced chemical equation has the total number of each kind of atom or ion in the reactants equal to the total number of the same kind of atom or ion in the products • When more than one molecule or formula unit is required, a coefficient is used
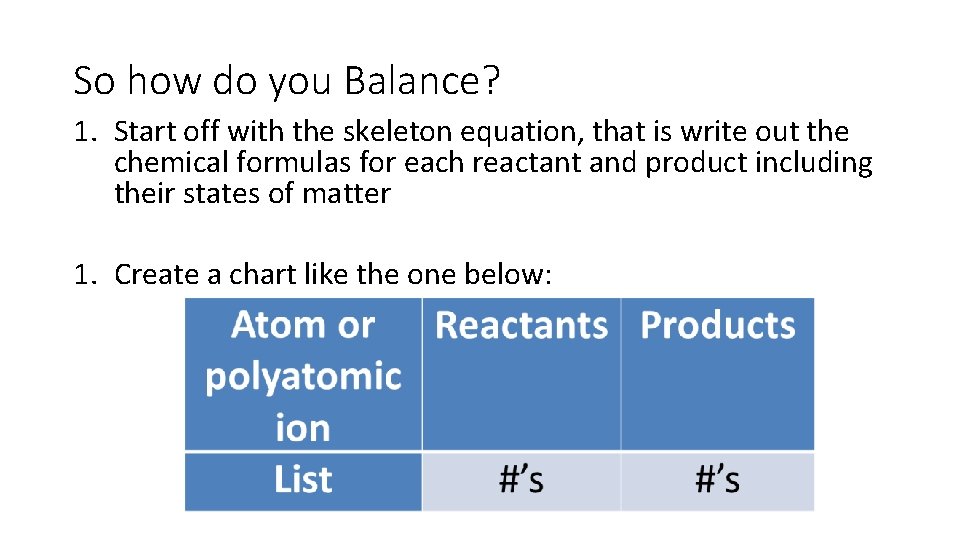
So how do you Balance? 1. Start off with the skeleton equation, that is write out the chemical formulas for each reactant and product including their states of matter 1. Create a chart like the one below:

How do you balance? 3. Compare the numbers of each atom or polyatomic ion from the reactant and product side: • if the numbers are equal to each other then the atom or polyatomic ion is conserved and you write a 1 as the coefficient in front of the appropriate chemical • if there is a discrepancy you need to determine a number that is common to both and this number is written as the coefficient in front of the appropriate chemical

How do you balance? 4. Once a coefficient is written you must start over rechecking all the entities involved and if they do not balance then you must repeat step . 5. You are done when all the reactant amounts equal the product amounts.

Tips for Balancing Equations: • • Make sure all chemical formulas are correct before you start Polyatomic ions remain intact and are balanced as a single unit Balance any atoms that are present in more than 2 substance last If a fractional coefficient is needed to balance and atom, multiply all coefficients by the denominator of the fraction to get integers (whole numbers) • Sometimes you may need to start over • Eventually you will get it! Don’t give up!
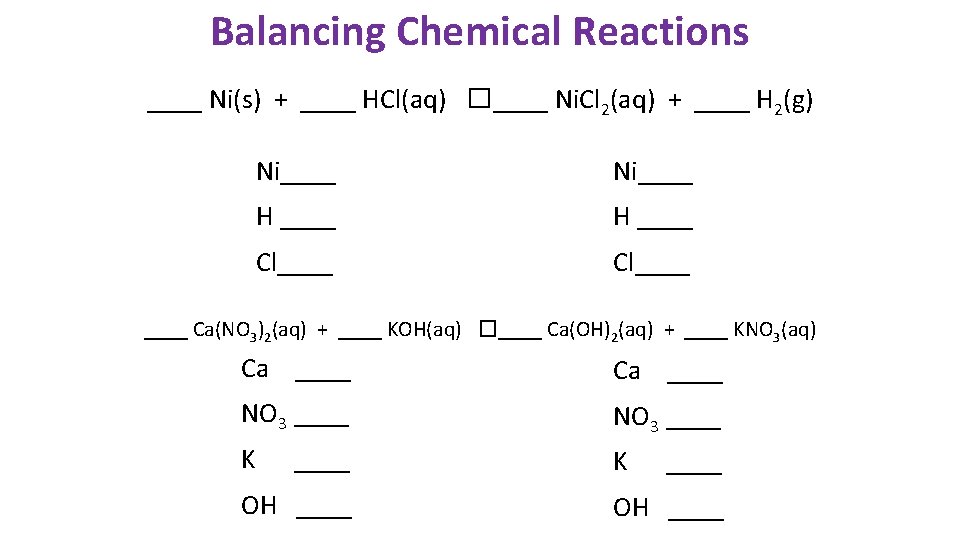
Balancing Chemical Reactions ____ Ni(s) + ____ HCl(aq) �____ Ni. Cl 2(aq) + ____ H 2(g) Ni____ H ____ Cl____ Ca(NO 3)2(aq) + ____ KOH(aq) �____ Ca(OH)2(aq) + ____ KNO 3(aq) Ca ____ NO 3 ____ K K ____ OH ____
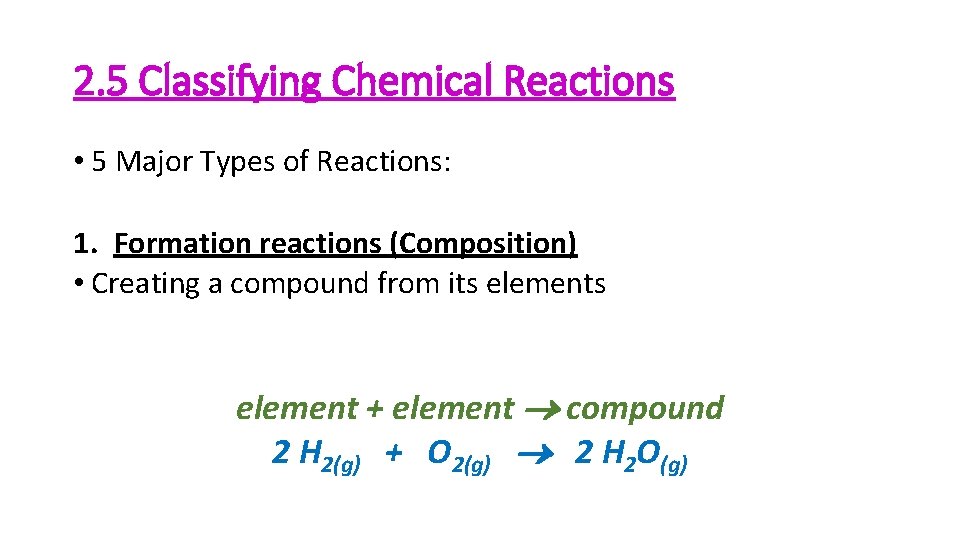
2. 5 Classifying Chemical Reactions • 5 Major Types of Reactions: 1. Formation reactions (Composition) • Creating a compound from its element + element compound 2 H 2(g) + O 2(g) 2 H 2 O(g)
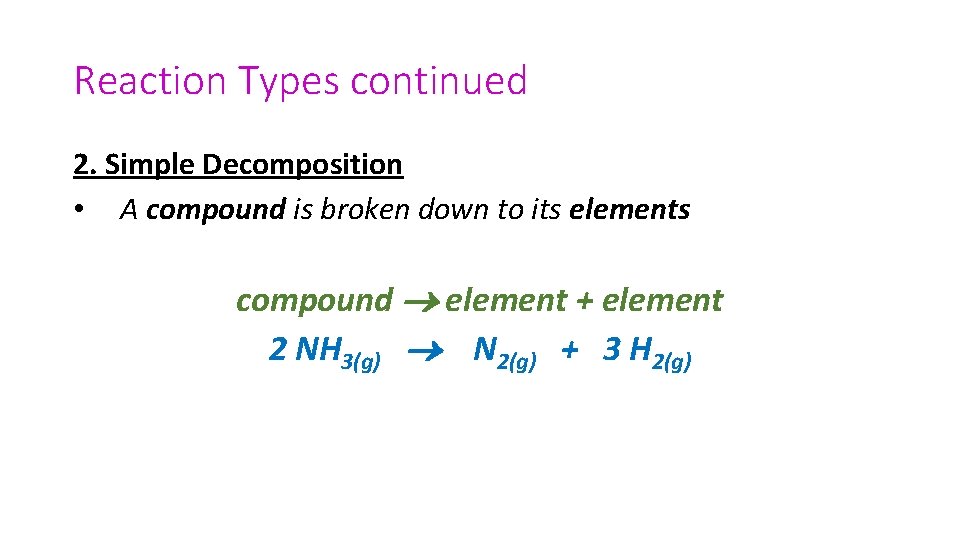
Reaction Types continued 2. Simple Decomposition • A compound is broken down to its elements compound element + element 2 NH 3(g) N 2(g) + 3 H 2(g)
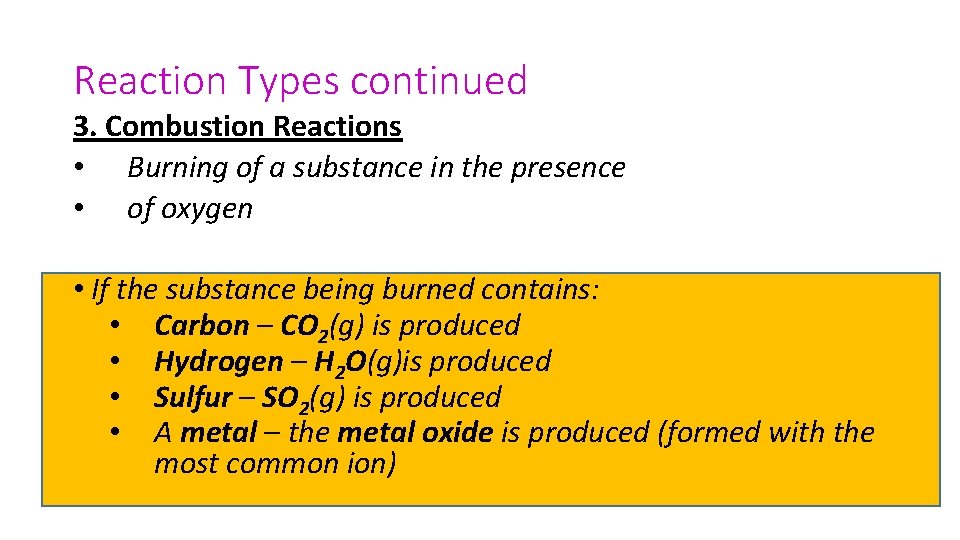
Reaction Types continued 3. Combustion Reactions • Burning of a substance in the presence • of oxygen • If the substance being burned contains: • Carbon – CO 2(g) is produced • Hydrogen – H 2 O(g)is produced • Sulfur – SO 2(g) is produced • A metal – the metal oxide is produced (formed with the most common ion)
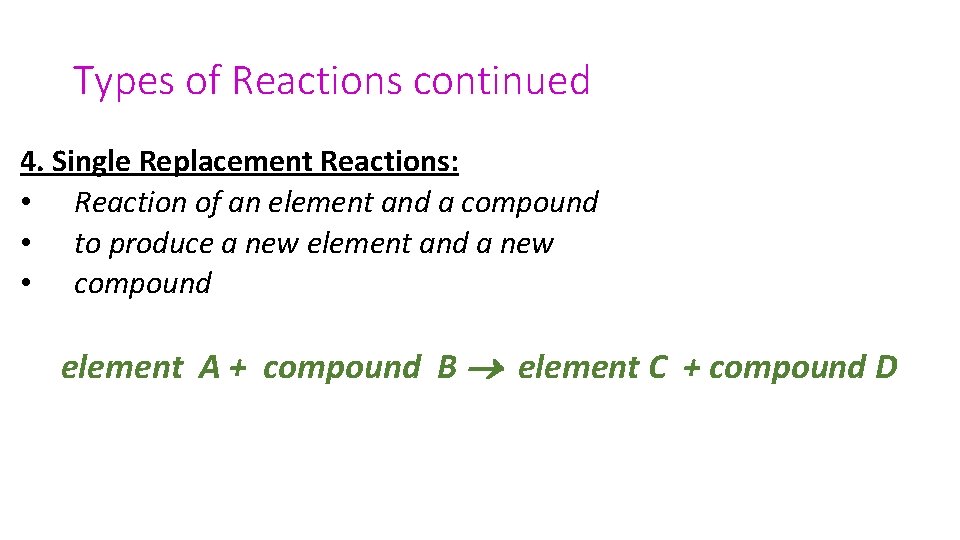
Types of Reactions continued 4. Single Replacement Reactions: • Reaction of an element and a compound • to produce a new element and a new • compound element A + compound B element C + compound D

Types of Reactions continued 4. Single Replacement Reactions: • They follow a very simple rule: • metal elements will replace the cation • non-metal elements will replace the anion • With these types of reactions you should pay close attention to your states of matter (solubility table)
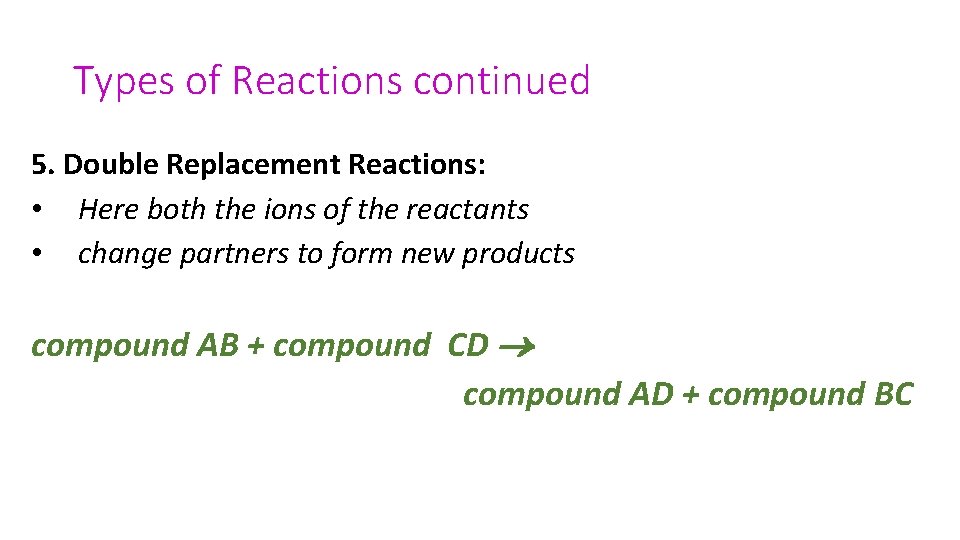
Types of Reactions continued 5. Double Replacement Reactions: • Here both the ions of the reactants • change partners to form new products compound AB + compound CD compound AD + compound BC

• 5. Double Replacement Reactions: • They follow a very simple rule: • cations will exchange their partners • With these types of reactions you should pay close attention to your states of matter (solubility table)

2. 4 Chemical Amount Significant Digits, Molar Mass and Moles

Significant Digits • These are the reliable digits reported in a measurement, keeping in mind that the last digit of a measurement is considered to be an estimated digit, but still significant. • There a few rules that we will apply in determining the number of significant digits a value has:

Significant Digits • Digits 1 -9 (NOT 0) are ALWAYS significant, no matter where they are in the value • The position of zeroes in a number determines whether or not they are significant Zeroes in front are NEVER significant Zeroes between digits ARE significant Zeroes at the end ARE significant
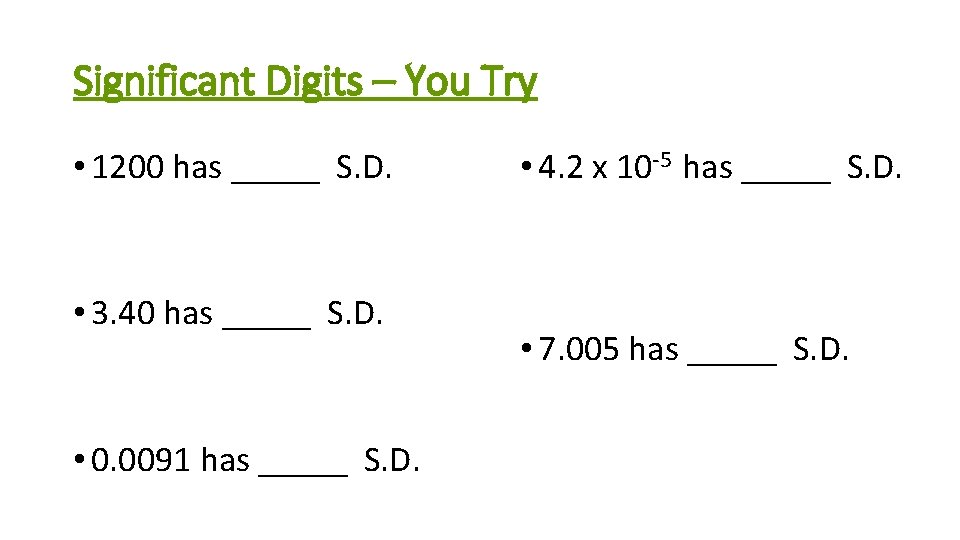
Significant Digits – You Try • 1200 has _____ S. D. • 3. 40 has _____ S. D. • 0. 0091 has _____ S. D. • 4. 2 x 10 -5 has _____ S. D. • 7. 005 has _____ S. D.
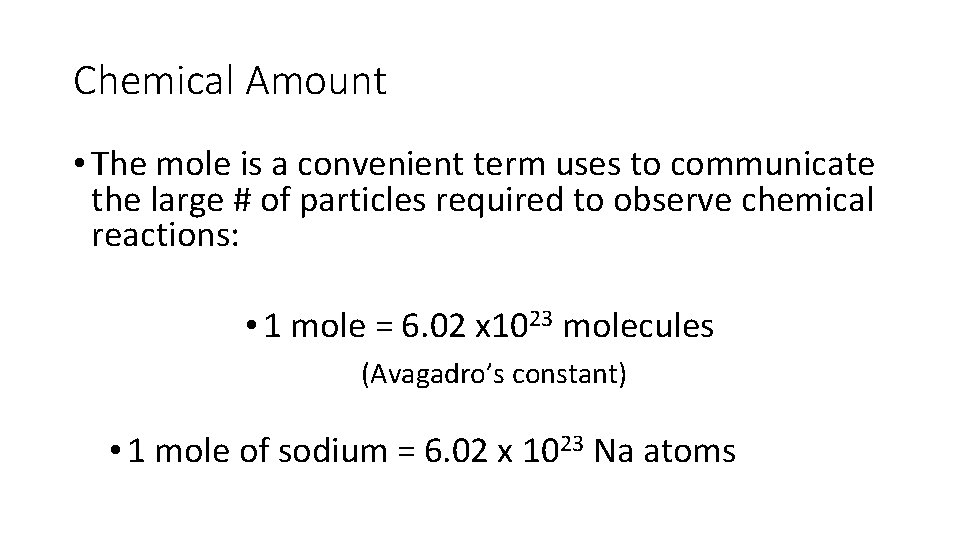
Chemical Amount • The mole is a convenient term uses to communicate the large # of particles required to observe chemical reactions: • 1 mole = 6. 02 x 1023 molecules (Avagadro’s constant) • 1 mole of sodium = 6. 02 x 1023 Na atoms

Molar Mass • The molar mass, M, of a substance is the mass of one mole of that substance • Unit is grams per mole (g/mol) • It is found by adding up the atomic molar masses of all the elements that make it up (and how many of each element)

Molar Mass • To calculate the molar mass: You need to write out the correct formula for the compound Locate atomic mass for each element using the periodic table Total all the atomic masses together to get the molar mass of the compound
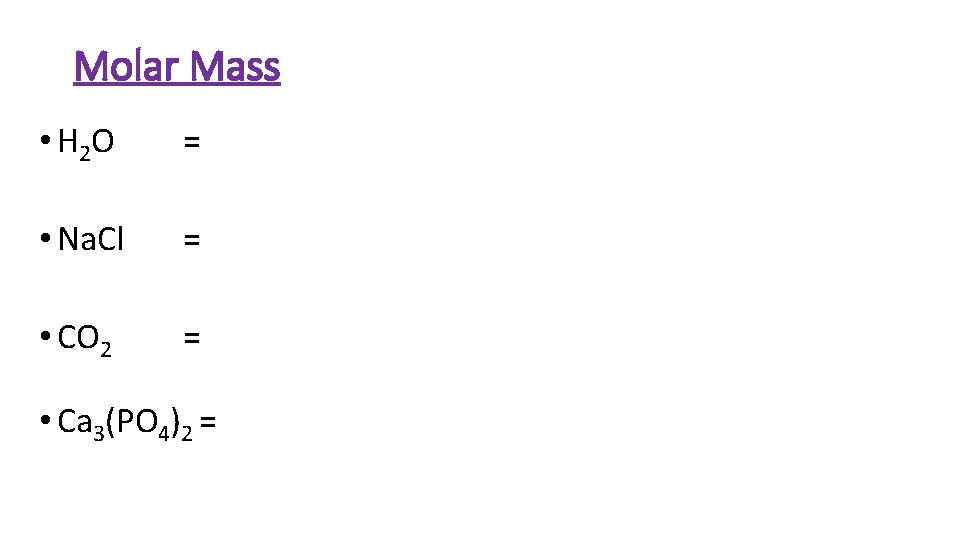
Molar Mass • H 2 O = • Na. Cl = • CO 2 = • Ca 3(PO 4)2 =

Molar Mass – You Try • For hydrates: • You multiple the molar mass of water by the coefficient stated then you add this value to the molar mass of the rest of the compound • Ex: Ca(OH)2 • 6 H 2 O
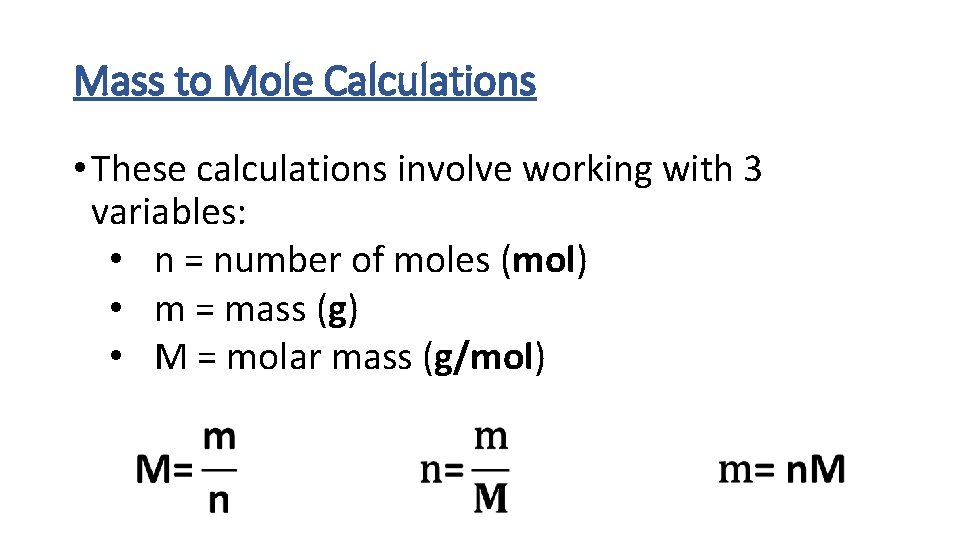
Mass to Mole Calculations • These calculations involve working with 3 variables: • n = number of moles (mol) • m = mass (g) • M = molar mass (g/mol)

Mass to Mole Calculations • Solving for n so you need to know the other 2 variables, m and M How many moles are in 6. 0 g or CO 2? • m = 6. 0 g • M needs to be calculated • n is needed
Mass to Mole Calculations Now to calculate the unknown value we use the STRING (or line) approach • No formulas to memorize if you remember how to do it • The value with one unit goes first • You ALWAYS multiply • Units cancel our when the some one appears in the numerator and denominator
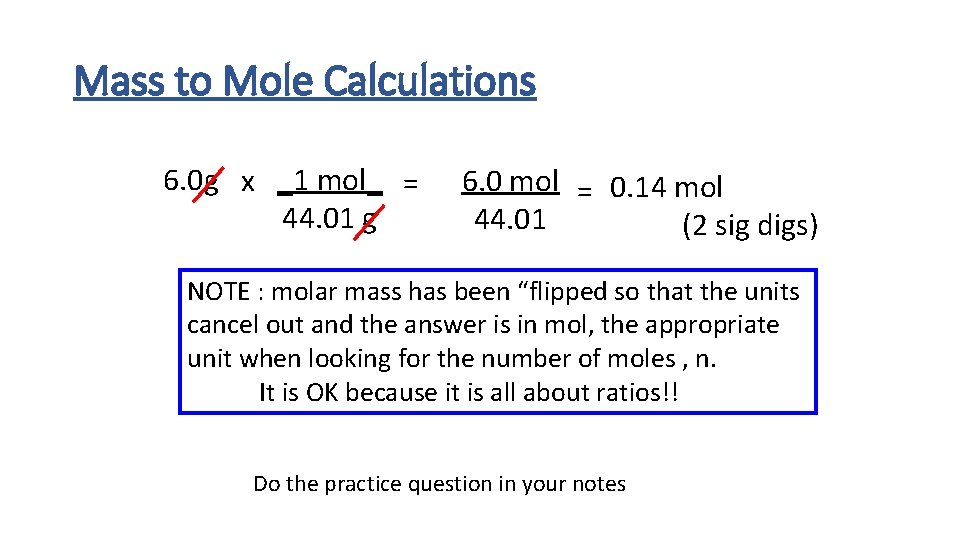
Mass to Mole Calculations 6. 0 g x _1 mol_ = 44. 01 g 6. 0 mol = 0. 14 mol 44. 01 (2 sig digs) NOTE : molar mass has been “flipped so that the units cancel out and the answer is in mol, the appropriate unit when looking for the number of moles , n. It is OK because it is all about ratios!! Do the practice question in your notes
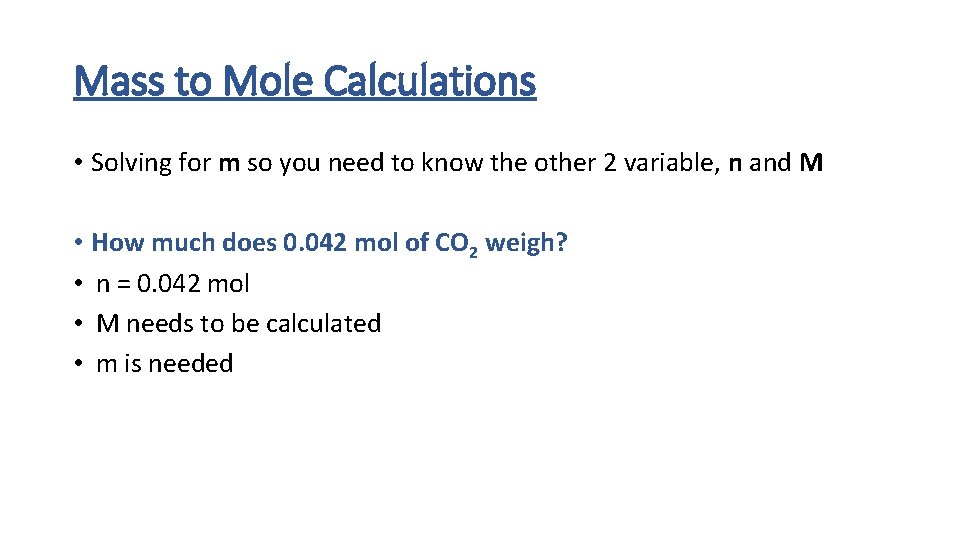
Mass to Mole Calculations • Solving for m so you need to know the other 2 variable, n and M • How much does 0. 042 mol of CO 2 weigh? • n = 0. 042 mol • M needs to be calculated • m is needed
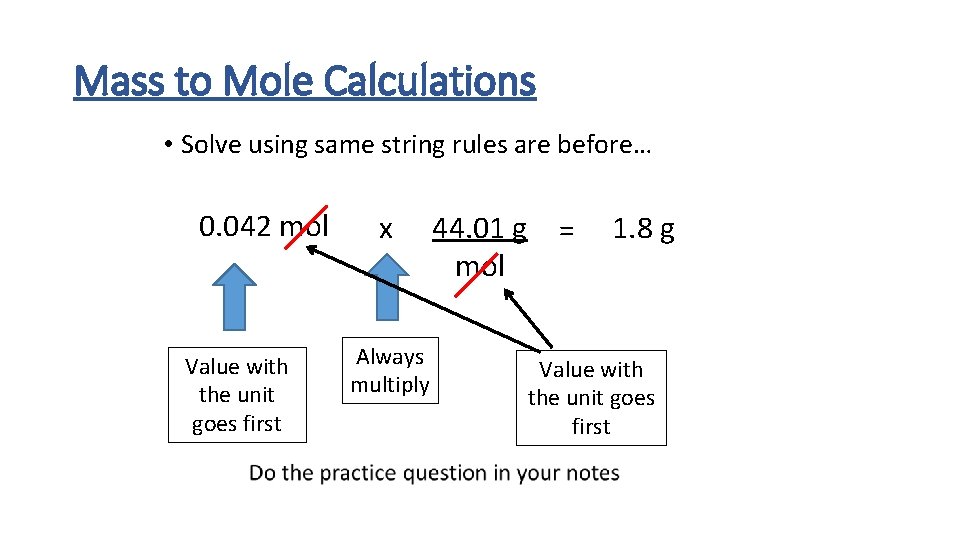
Mass to Mole Calculations • Solve using same string rules are before… 0. 042 mol Value with the unit goes first x Always multiply 44. 01 g = mol 1. 8 g Value with the unit goes first
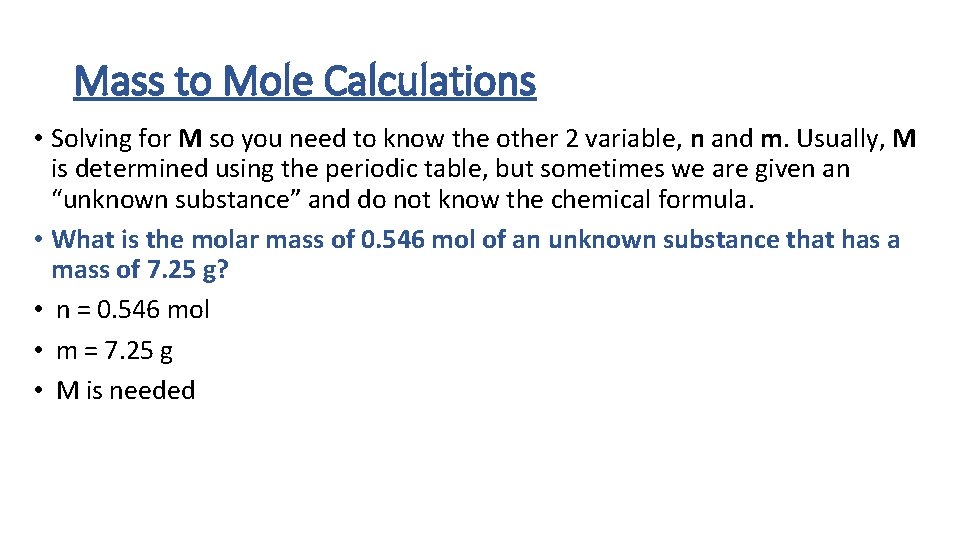
Mass to Mole Calculations • Solving for M so you need to know the other 2 variable, n and m. Usually, M is determined using the periodic table, but sometimes we are given an “unknown substance” and do not know the chemical formula. • What is the molar mass of 0. 546 mol of an unknown substance that has a mass of 7. 25 g? • n = 0. 546 mol • m = 7. 25 g • M is needed
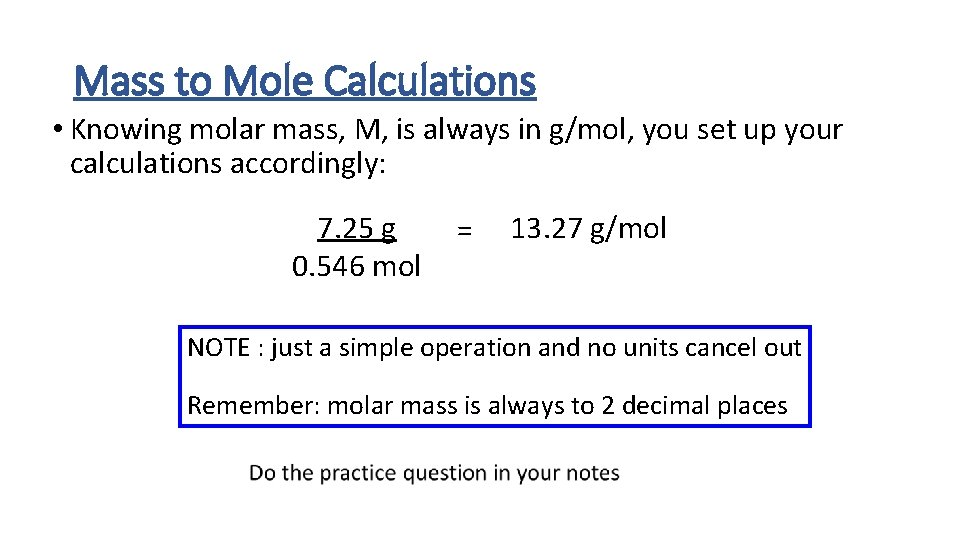
Mass to Mole Calculations • Knowing molar mass, M, is always in g/mol, you set up your calculations accordingly: 7. 25 g 0. 546 mol = 13. 27 g/mol NOTE : just a simple operation and no units cancel out Remember: molar mass is always to 2 decimal places
- Slides: 44