Chemical Reactions Chapter 11 What is a Chemical














- Slides: 17

Chemical Reactions Chapter 11

What is a Chemical Reaction? n The process in which atoms of one or more substances are rearranged to form new different substances

Evidence of Chemical Reactions Temperature change (hot or cold) n Light n Color change n Odor n Gas bubbles n Appearance of solids n

Reactants and Products A+B C+D

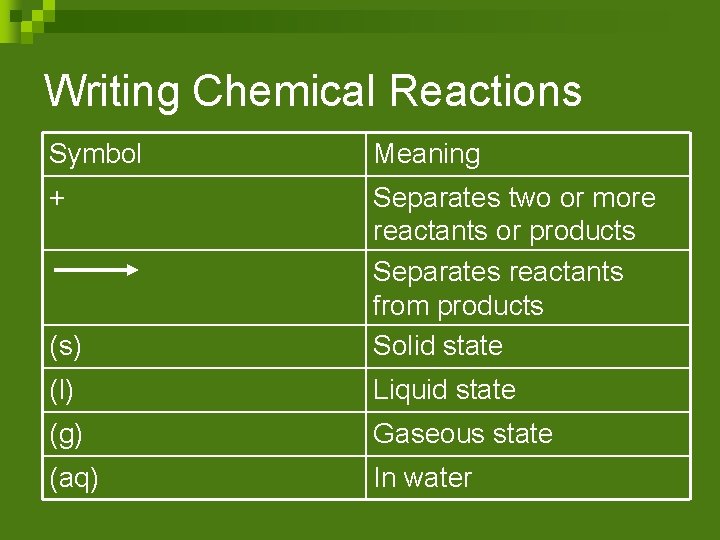
Writing Chemical Reactions Symbol Meaning + (s) Separates two or more reactants or products Separates reactants from products Solid state (l) Liquid state (g) Gaseous state (aq) In water

Word Equations n Use the names to show a reactions Ex: n Iron (s) + Chlorine (g) Iron (III) Chloride (s) n

Skeleton Equation Uses symbols to show reaction n Ex: n Iron (s) + Chlorine (g) Iron (III) Chloride (s) n Would become n Fe (s) + Cl 2 (g) Fe. Cl 3 (s) n

Law of Conservation of Mass You cannot create nor destroy mass n After a chemical reaction, no matter can be made and no matter can be destroyed n Mass Reactants = Mass Products n

Example Problem H 2 + Br 2 HBr Mass reactants = Mass Products =

Balancing Equations n To satisfy the law of conservation of mass, you have to balance equations to make the mass of the reactants = mass products

Adding Coefficients Have to make the same number of atoms of each element on both sides of the equation H 2 + Br 2 2 HBr Coefficients multiply every atom in the compound or molecule

Classifying Chemical RXN’s n You can classify chemical reactions in one of five categories ¨ Synthesis ¨ Decomposition ¨ Combustion ¨ Single Replacement ¨ Double Replacement

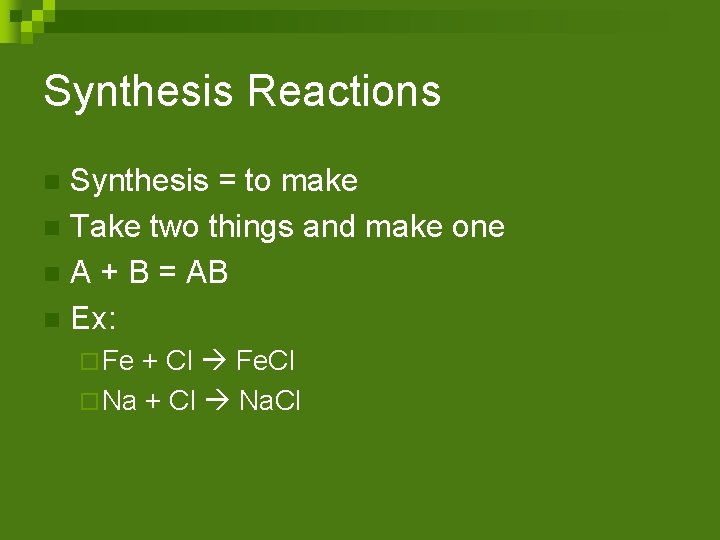
Synthesis Reactions Synthesis = to make n Take two things and make one n A + B = AB n Ex: n ¨ Fe + Cl Fe. Cl ¨ Na + Cl Na. Cl

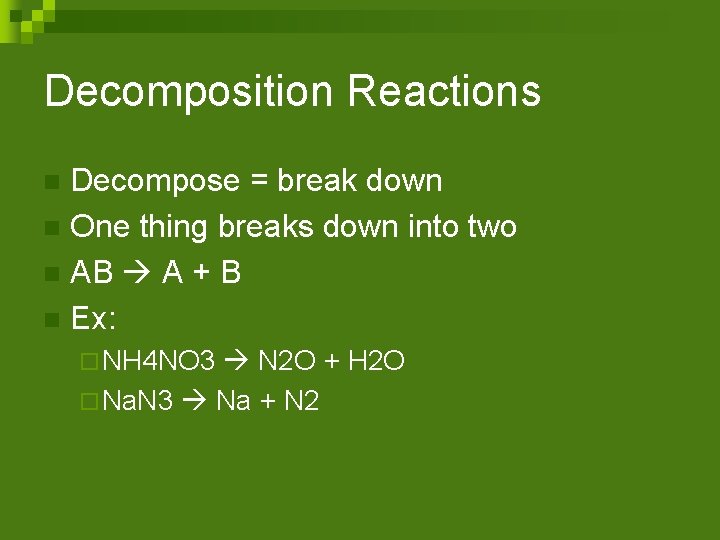
Decomposition Reactions Decompose = break down n One thing breaks down into two n AB A + B n Ex: n ¨ NH 4 NO 3 N 2 O + H 2 O ¨ Na. N 3 Na + N 2

Combustion Reactions where you have combustion ¨ Engines, gas grills, gas fireplaces, burning things Oxygen is always a reactant and CO 2 & H 2 O are always the products n Ex: n ¨ CH 4 + O 2 CO 2 + H 2 O

Single Replacement Reactions One Ion switches places with another n A + BX AX + B n Ex: n ¨ Cu + Ag. NO 3 Cu. NO 3 + Ag ¨ F 2 + Na. Br Na. F + Br 2

Double Replacement Reactions Two ions switch places with each other n AX + BY AY + BX n Ex: n ¨ Ca(OH) + HCl Ca. Cl + HOH ¨ Na. OH + Cu. Cl Na. Cl + Cu. OH ¨ KCN + HBr KBr + HCN