Chemical Reactions Chapter 11 Introduction Learn how to






















- Slides: 62

Chemical Reactions Chapter 11

Introduction • Learn how to write word descriptions of chemical reactions. • The word descriptions can then be translated into symbols. • Balance the chemical equations for chemical reactions. • Learn about the different reaction types and spot the patterns in each reaction type so that we can make predictions of what products will form given a set of reactants. • Learn about what an aqueous environment is and the reactions that take place there.

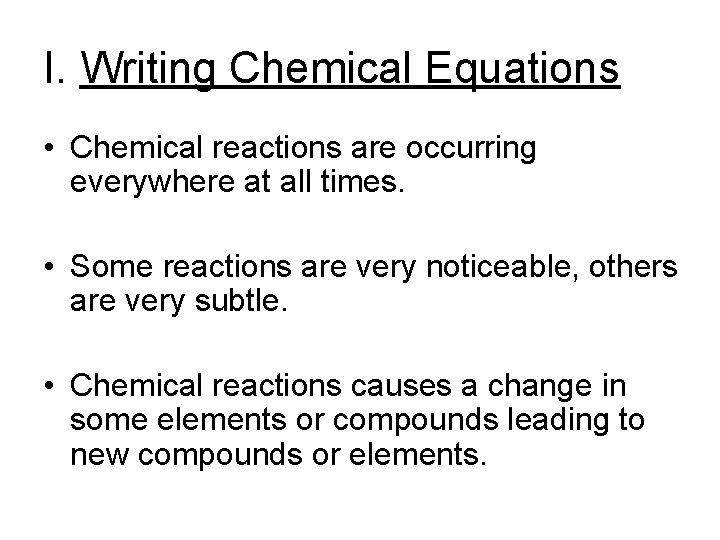
Describing Chemical Reactions (Section 11. 1) • Writing Chemical Equations • Balancing Chemical Equations 2 H 2 + O 2 2 H 2 O

I. Writing Chemical Equations • Chemical reactions are occurring everywhere at all times. • Some reactions are very noticeable, others are very subtle. • Chemical reactions causes a change in some elements or compounds leading to new compounds or elements.

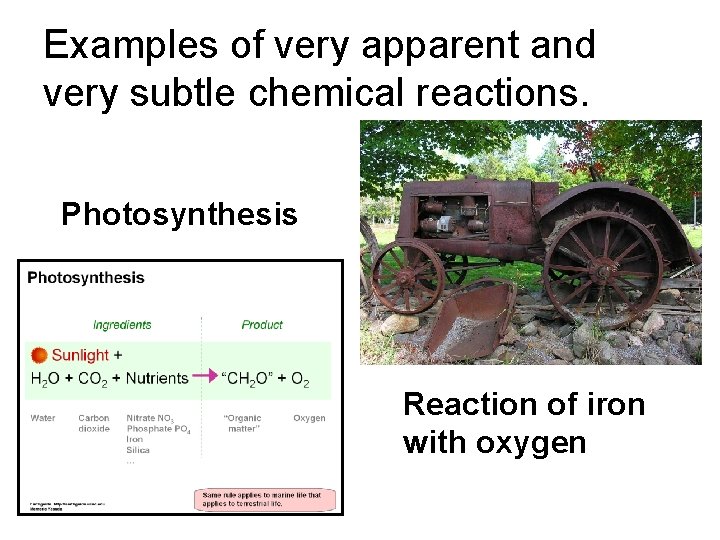
Examples of very apparent and very subtle chemical reactions. Photosynthesis Reaction of iron with oxygen

Word equations are shorter methods of representing a chemical reaction. • Reactants on the left • Products on the right • An arrow separating them pointing towards the products. (“yields, ” “gives, ” “reacts to produce”) reactants products

How could we describe the formation of rust? • “Iron reacts with oxygen to give iron(III) oxide. ” • Iron + oxygen iron(III) oxide

Writing word equations 1. Write the name(s) of the reactant(s) to the left of an arrow. Separate the reactants using a plus sign. 2. Write the name(s) of the product(s) to the right of an arrow. Separate the products using a plus sign.

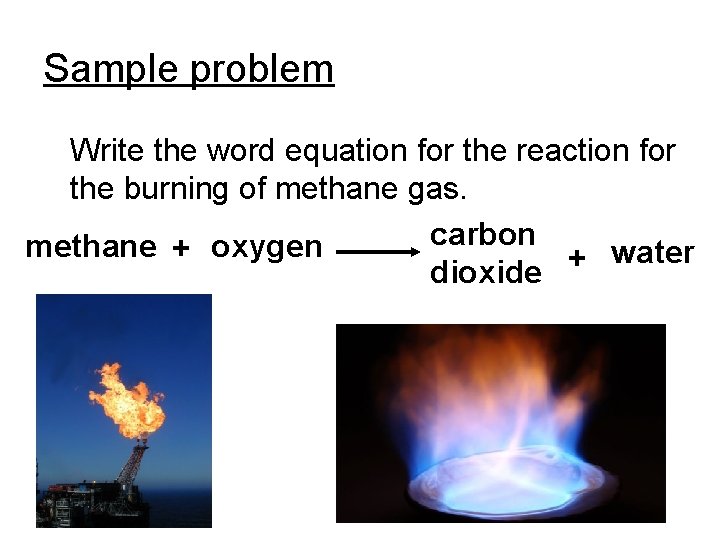
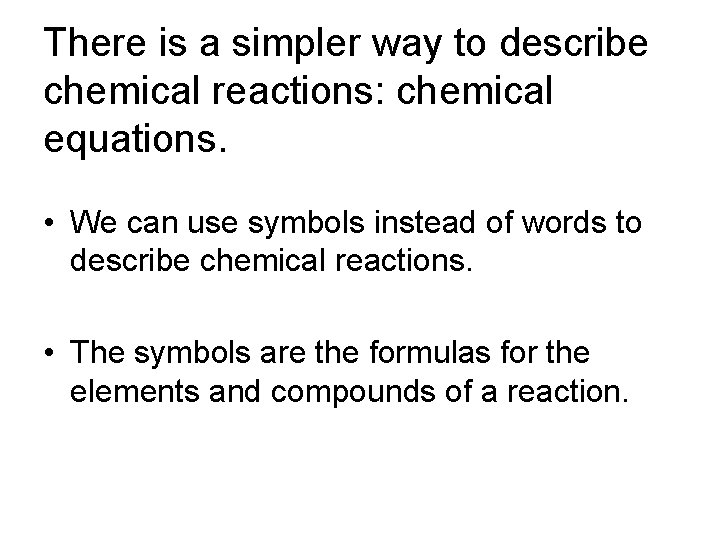
Sample problem Write the word equation for the reaction for the burning of methane gas. carbon methane + oxygen water + dioxide

There is a simpler way to describe chemical reactions: chemical equations. • We can use symbols instead of words to describe chemical reactions. • The symbols are the formulas for the elements and compounds of a reaction.

Skeleton equations do not indicate the relative amounts of the reactants and products of a reaction. • Fe + O 2 Fe 2 O 3 – The atoms are not balanced in this equation. – This kind of equation gives only the reactants and products of the reaction. – Writing this equation simply requires you put all the reactants on the left side of the arrow and all the products on the right.

Indicating the physical states of the reactants and products in a chemical equation. • It is helpful to have the physical states of the components of a reaction indicated in a chemical equation. • solids = s • liquids = l • gases = g • aqueous = aq (compound dissolved in water)

Other information can be included in the chemical equation as well. • Information such as a reaction’s need of a catalyst or of heat can be included above the arrow. • Catalyst: A substance that speeds up a reaction but does not change during the course of the reaction. • H 2 O 2(aq) Mn. O 2 H 2 O(l) + O 2(g)

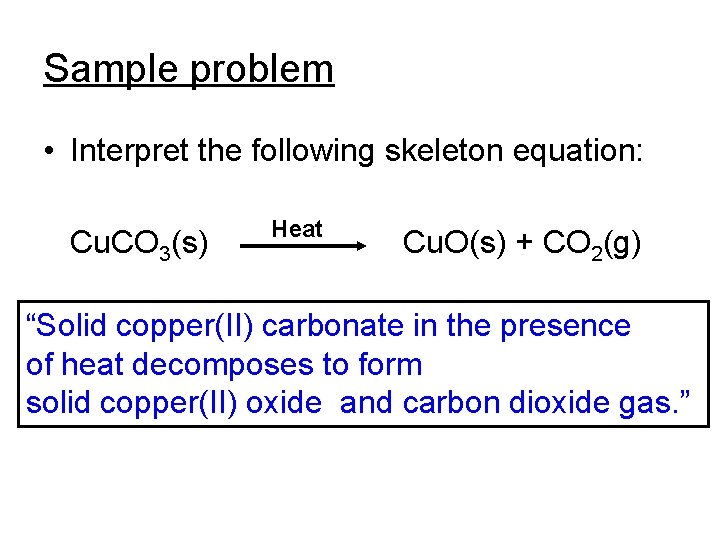
Sample problem • Interpret the following skeleton equation: Cu. CO 3(s) Heat Cu. O(s) + CO 2(g) “Solid copper(II) carbonate in the presence of heat decomposes to form solid copper(II) oxide and carbon dioxide gas. ”

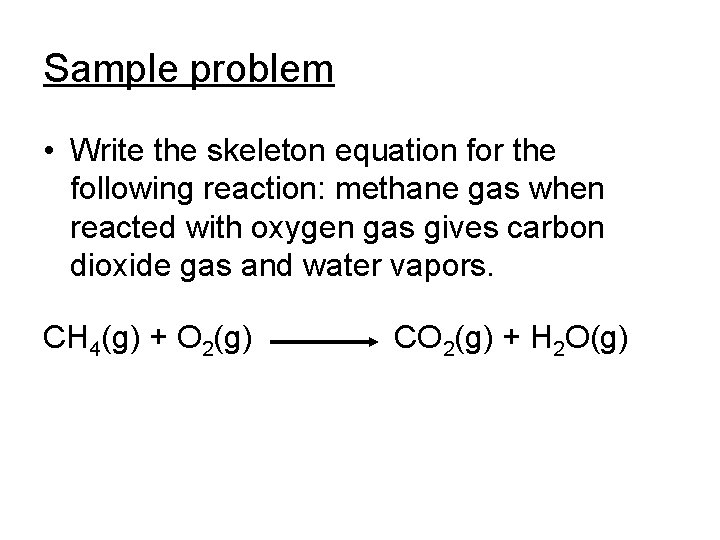
Sample problem • Write the skeleton equation for the following reaction: methane gas when reacted with oxygen gas gives carbon dioxide gas and water vapors. CH 4(g) + O 2(g) CO 2(g) + H 2 O(g)

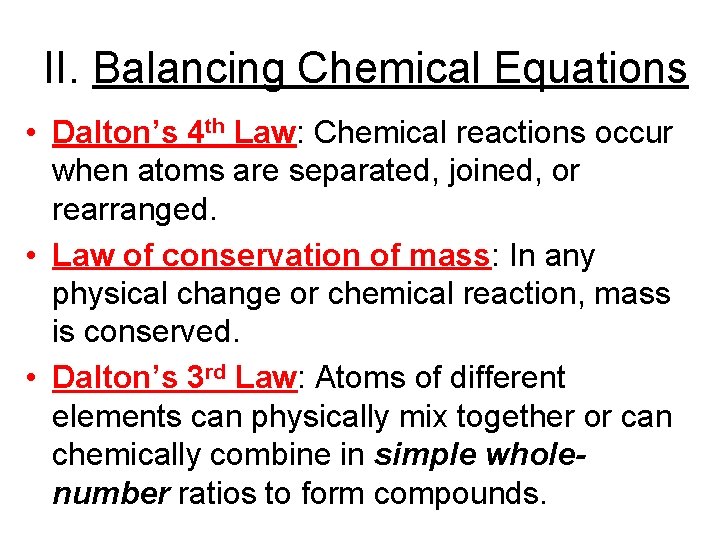
II. Balancing Chemical Equations • Dalton’s 4 th Law: Chemical reactions occur when atoms are separated, joined, or rearranged. • Law of conservation of mass: In any physical change or chemical reaction, mass is conserved. • Dalton’s 3 rd Law: Atoms of different elements can physically mix together or can chemically combine in simple wholenumber ratios to form compounds.

Chemical reactions must have the same number and kinds of atoms at the end at the start of the reaction. 2 K(s) + 2 H 2 O(l) → 2 KOH(aq) + H 2(g) C 12 H 22 O 11(s)+ H 2 SO 4 (aq) → 12 C (s) + 11 H 2 O(g)

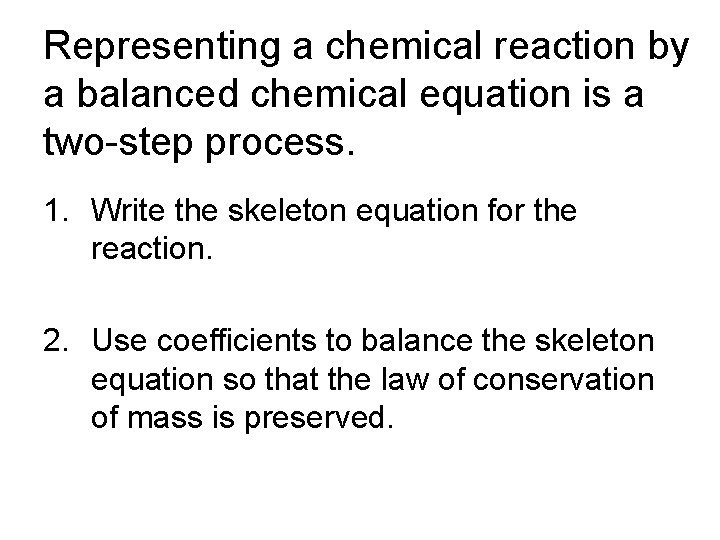
Representing a chemical reaction by a balanced chemical equation is a two-step process. 1. Write the skeleton equation for the reaction. 2. Use coefficients to balance the skeleton equation so that the law of conservation of mass is preserved.

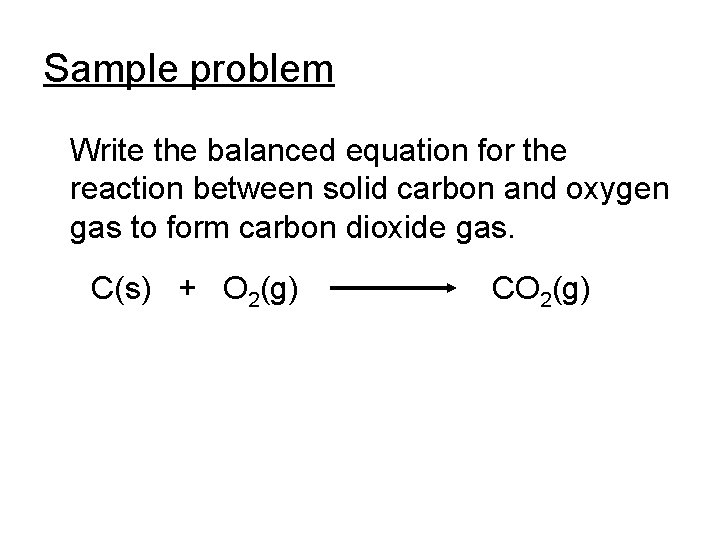
Sample problem Write the balanced equation for the reaction between solid carbon and oxygen gas to form carbon dioxide gas. C(s) + O 2(g) CO 2(g)

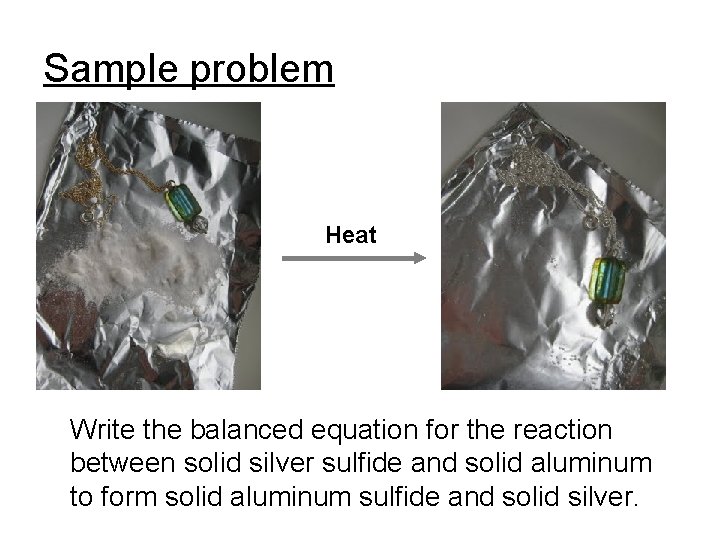
Sample problem Heat Write the balanced equation for the reaction between solid silver sulfide and solid aluminum to form solid aluminum sulfide and solid silver.

Types of Chemical Reactions (Section 11. 2) • Classifying Reactions • Predicting the Products of a Chemical Reaction

I. Classifying Reactions • There are too many chemical reactions to memorize. • Grouping reactions by certain characteristics will help in identifying them. • There are five general types of reactions. – Not all chemical reactions fit neatly into one of these categories and some can be grouped in two.

There are five basic categories of reactions. Decomposition reactions Single-replacement reactions Combination reactions Double-replacement reactions Combustion reactions

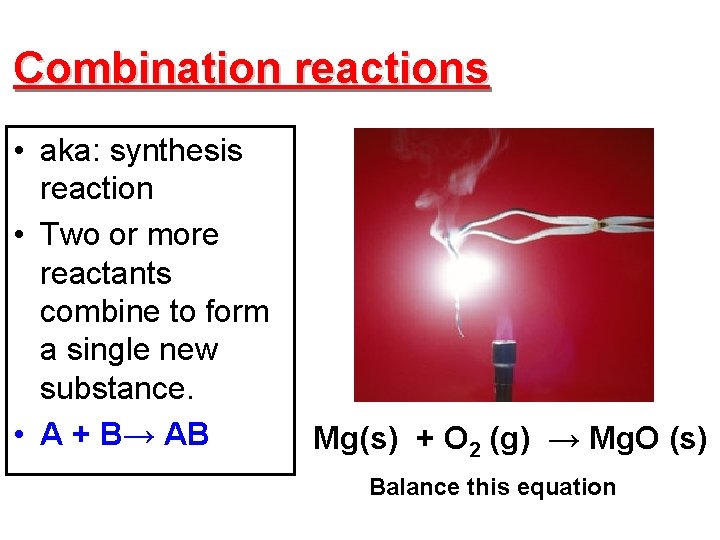
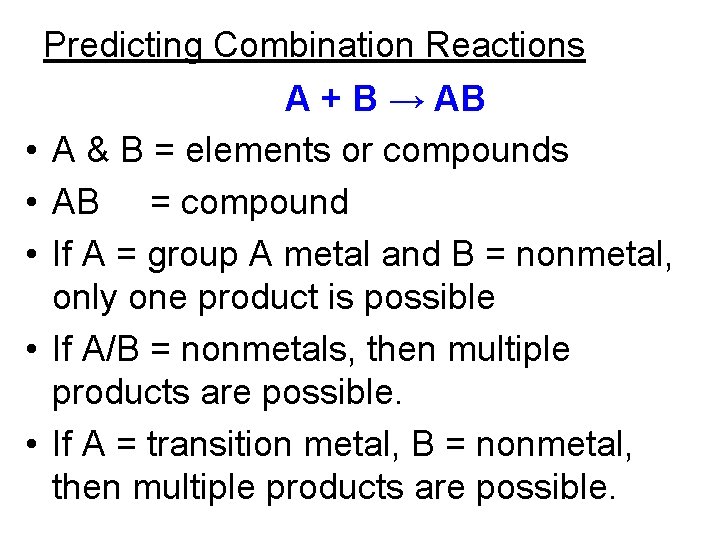
Combination reactions • aka: synthesis reaction • Two or more reactants combine to form a single new substance. • A + B→ AB Mg(s) + O 2 (g) → Mg. O (s) Balance this equation

Characteristics of combination reaction. • 2 elements or two compounds combine together to form a single, new compound. • Group A metals and nonmetals form ionic compounds. • Two nonmetals can combine to form more than one type of product. • Transition metals and nonmetals can form more than one type of ionic compound.

Group A metals and nonmetals form ionic compounds. Na(s) + Cl 2(g) → Na. Cl Balance this equation.

Two nonmetals can combine to form more than one type of product. • S(s) + O 2 → SO 2(g) – What’s the name of this product? • 2 S(s) + 3 O 2(g) → 2 SO 3(g) – What’s the name of this product?

Transition metals and nonmetals can form more than one type of ionic compound. Fe (s) + S (s) → Fe. S (s) 2 Fe (s) + 3 S (s) → Fe 2 S 3 (s) Name the products in each of the reactions.

Sample problem Copper and sulfur are reactants in a combination reaction. Complete the equation for following reaction: Cu(s) + S(s) → (two reactions possible)

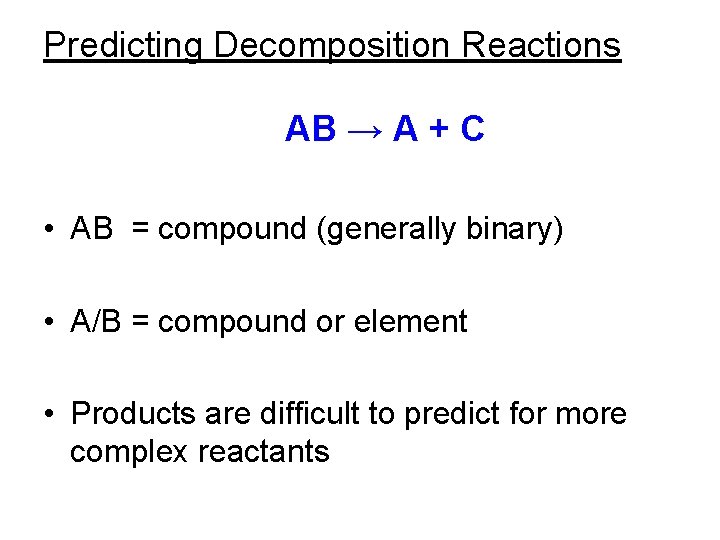
Decomposition reactions. • A chemical change in which a single compound breaks down into two or more simpler products. • AB → A + C Hg. O (s) → Hg(l) + O 2(g) (s) Balance this equation.

Characteristics of decomposition reactions. • These reactions involve only one reactant and two or more products. • The products can be simpler compounds, elements, or a combination of both. • It’s difficult to predict all the products of a decomposition reaction. • Most decomposition reactions require energy in the form of heat, light, or electricity.

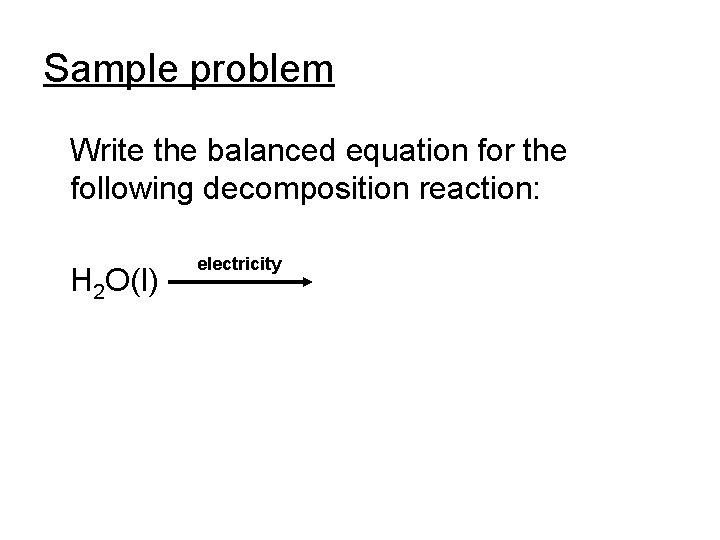
Sample problem Write the balanced equation for the following decomposition reaction: H 2 O(l) electricity

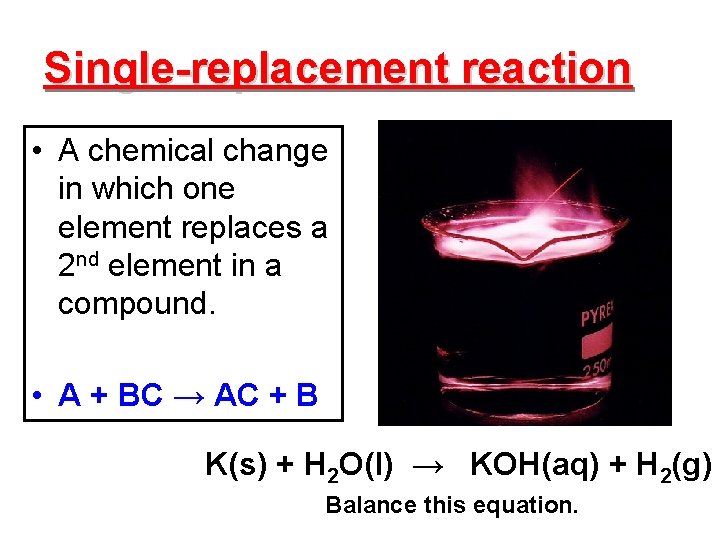
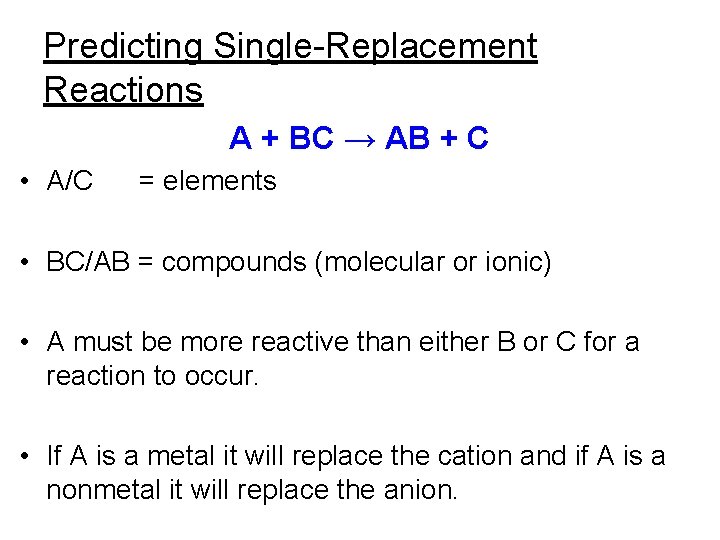
Single-replacement reaction • A chemical change in which one element replaces a 2 nd element in a compound. • A + BC → AC + B K(s) + H 2 O(l) → KOH(aq) + H 2(g) Balance this equation.

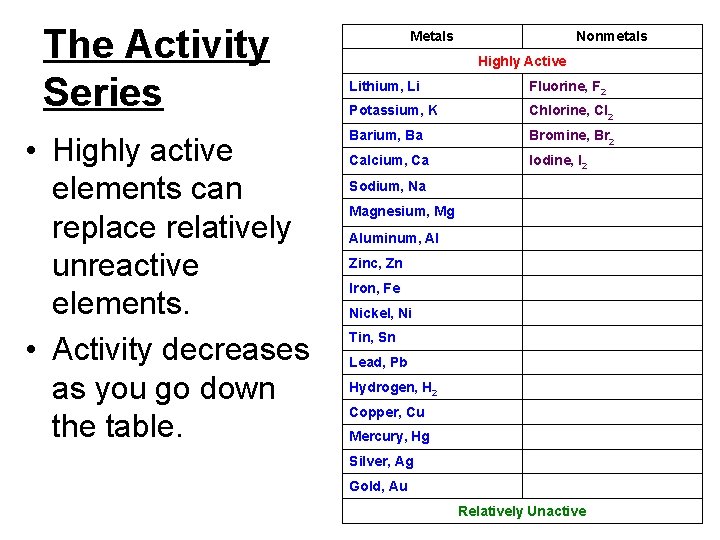
Characteristics of single-replacement reactions. • ID this reaction by noting that an element and a compound appear in both the reactants and products. • The activity series helps to determine if one element can replace another in a compound. – The replacement potential of an element depends upon its relative reactivity.

The Activity Series • Highly active elements can replace relatively unreactive elements. • Activity decreases as you go down the table. Metals Nonmetals Highly Active Lithium, Li Fluorine, F 2 Potassium, K Chlorine, Cl 2 Barium, Ba Bromine, Br 2 Calcium, Ca Iodine, I 2 Sodium, Na Magnesium, Mg Aluminum, Al Zinc, Zn Iron, Fe Nickel, Ni Tin, Sn Lead, Pb Hydrogen, H 2 Copper, Cu Mercury, Hg Silver, Ag Gold, Au Relatively Unactive

Sample problem Using Table 11. 2 on p. 333, describe what would happen when solid zinc is reacted with copper(II) nitrate.

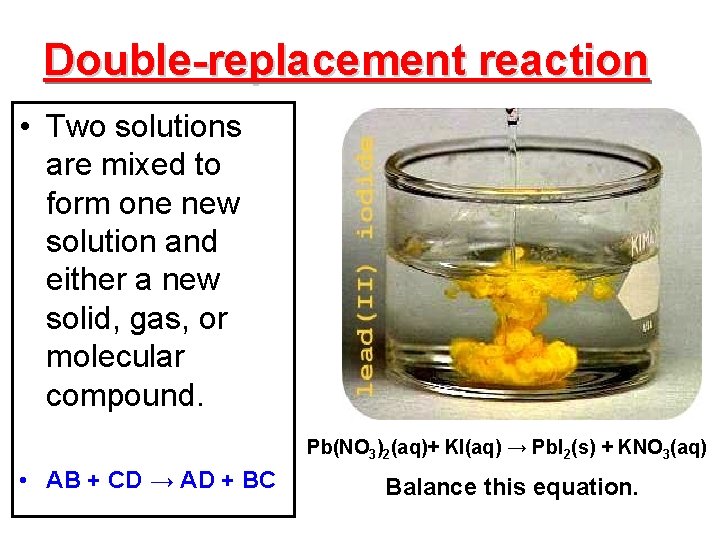
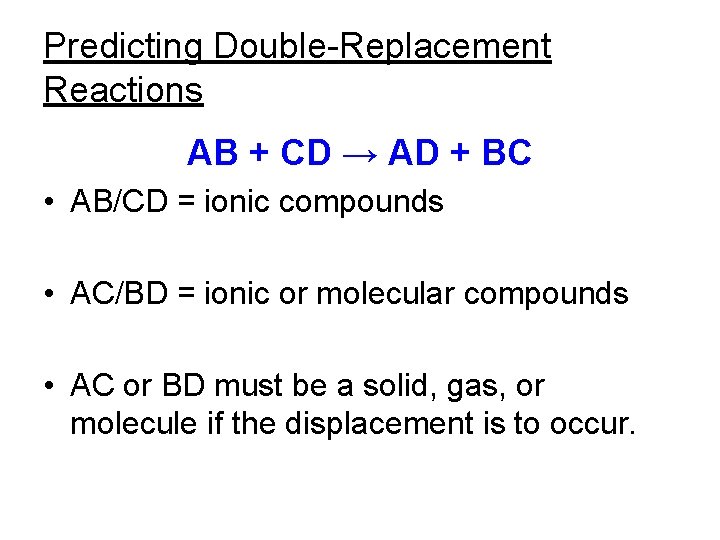
Double-replacement reaction • Two solutions are mixed to form one new solution and either a new solid, gas, or molecular compound. Pb(NO 3)2(aq)+ KI(aq) → Pb. I 2(s) + KNO 3(aq) • AB + CD → AD + BC Balance this equation.

Characteristics of double replacement reactions • aka: “double displacement” reaction. • The solutions are ionic and “soluble” in water. • The ionic solutions exchange cations. • The products are solids known as precipitates, gases, or molecular compounds.

Sample problem If cadmium sulfide is a solid, decide whether the reaction between aqueous sodium sulfide and aqueous cadmium(II) nitrate will occur. Write an equation to show this reaction.

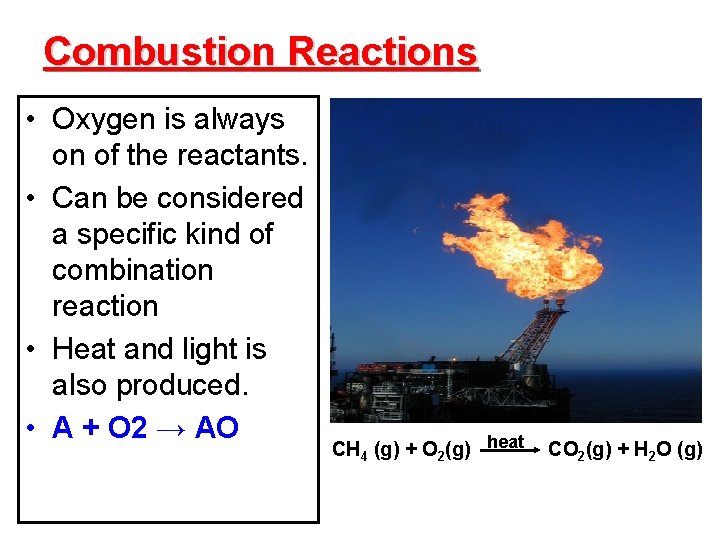
Combustion Reactions • Oxygen is always on of the reactants. • Can be considered a specific kind of combination reaction • Heat and light is also produced. • A + O 2 → AO CH 4 (g) + O 2(g) heat CO 2(g) + H 2 O (g)

Characteristics of combustion reactions • The other reactants in a combustion reaction may vary, but common ones involve hydrocarbons • Complete combustion of hydrocarbons produce CO 2(g) and H 2 O(g). Incomplete combustion of these reactants also produces CO(g). • Though heat and light are produced, heat is required to initiate the reaction.

Sample problem Predict the products for the complete combustion reaction of the hydrocarbon octane (C 8 H 18).

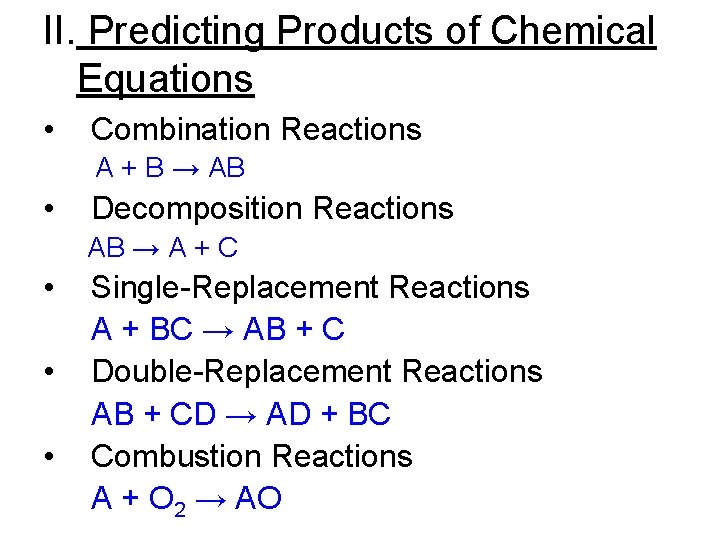
II. Predicting Products of Chemical Equations • Combination Reactions A + B → AB • Decomposition Reactions AB → A + C • • • Single-Replacement Reactions A + BC → AB + C Double-Replacement Reactions AB + CD → AD + BC Combustion Reactions A + O 2 → AO

Predicting Combination Reactions A + B → AB • A & B = elements or compounds • AB = compound • If A = group A metal and B = nonmetal, only one product is possible • If A/B = nonmetals, then multiple products are possible. • If A = transition metal, B = nonmetal, then multiple products are possible.

Predicting Decomposition Reactions AB → A + C • AB = compound (generally binary) • A/B = compound or element • Products are difficult to predict for more complex reactants

Predicting Single-Replacement Reactions A + BC → AB + C • A/C = elements • BC/AB = compounds (molecular or ionic) • A must be more reactive than either B or C for a reaction to occur. • If A is a metal it will replace the cation and if A is a nonmetal it will replace the anion.

Predicting Double-Replacement Reactions AB + CD → AD + BC • AB/CD = ionic compounds • AC/BD = ionic or molecular compounds • AC or BD must be a solid, gas, or molecule if the displacement is to occur.

Predicting Combustion Reactions A + O 2 → AO • A can be any compound or element; often A is a hydrocarbon. • Oxygen will always be the 2 nd reactant. • If A is a hydrocarbon the products will always be CO 2 and H 2 O. • This can be considered a special type of combination reaction.

Reactions in Aqueous Solutions (Section 11. 3) • Net Ionic Equations • Predicting the Formation of a Precipitate

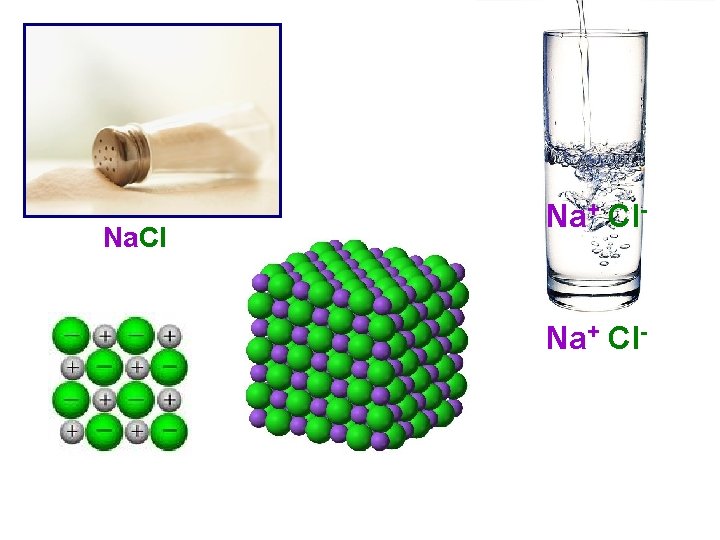
Na. Cl Na+ Cl-

I. Net Ionic Equations • Aqueous solutions (i. e. homogeneous mixtures) occur when a compound or element dissolves in water. • Many aqueous solutions contain a mixture of ions that could react with each other. • Many reactions occur between aqueous solutions containing ions. • An ionic equation shows the ions and nonionic compounds that are present in a reaction.

Ionic Equations • Two types: 1. Complete ionic equations 2. Net ionic equations • Complete Ionic Equation – An equation for a reaction that shows dissolved ionic compounds as dissociated free ions. • Net Ionic Equation – An equation for a reaction in solution that shows only those particles that are directly involved in the chemical change.

Writing Complete Ionic Equations Ag. NO 3(aq) + Na. Cl(aq) → Ag. Cl(s) + Na. NO 3(aq) Ag+ + NO 3 - + Na+ + Cl- Ag. Cl(s) + Na+ + NO 3 - Steps for writing complete ionic equations: 1. ) Check to make sure equation is balanced. 2. ) Identify the ionic compounds in aqueous solution. 3. ) For each compound write out the ions. Include the coefficient with each ion if one is present.

Sample problem Write the complete ionic equation for the following reaction. Pb(s) + Ag. NO 3(aq) → Ag(s) + Pb(NO 3)2(aq)

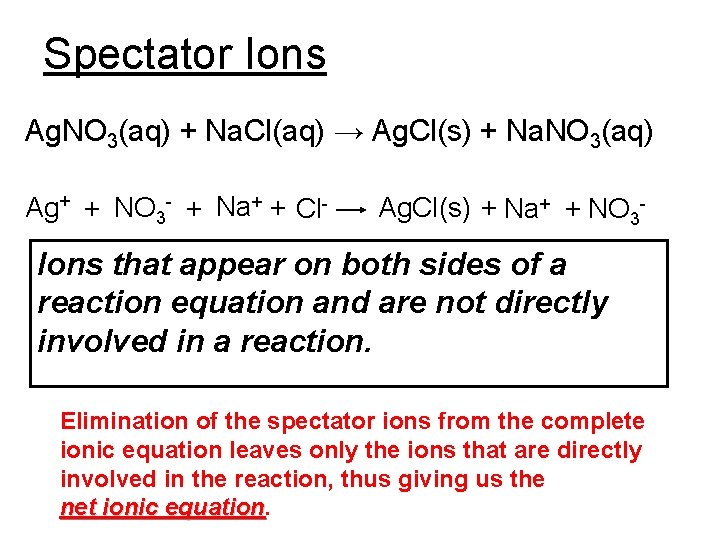
Spectator Ions Ag. NO 3(aq) + Na. Cl(aq) → Ag. Cl(s) + Na. NO 3(aq) Ag+ + NO 3 - + Na+ + Cl- Ag. Cl(s) + Na+ + NO 3 - Ions that appear on both sides of a reaction equation and are not directly involved in a reaction. Elimination of the spectator ions from the complete ionic equation leaves only the ions that are directly involved in the reaction, thus giving us the net ionic equation

Writing Net Ionic Equations 1. Write down the complete chemical equation. 2. Balance the equation. 3. If compounds are “aqueous” then separate them into their ions to get the complete ionic equation. 4. Eliminate all spectator ions with their coefficients if the have them. 5. Write down whatever is left over and this is the net ionic equation.

Sample problem Write the net ionic equation for the reaction between solid lead and aqueous silver nitrate to give solid silver and aqueous lead(II) nitrate.

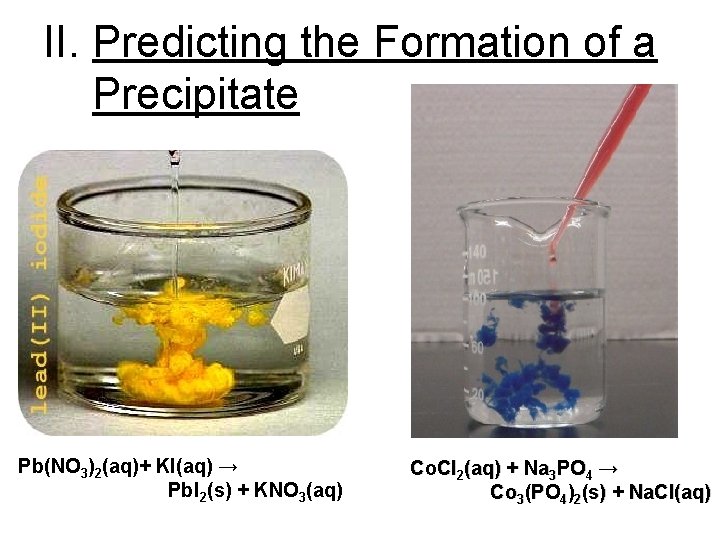
II. Predicting the Formation of a Precipitate Pb(NO 3)2(aq)+ KI(aq) → Pb. I 2(s) + KNO 3(aq) Co. Cl 2(aq) + Na 3 PO 4 → Co 3(PO 4)2(s) + Na. Cl(aq)

What is a precipitate? A precipitate is an insoluble ionic compound (i. e. insoluble salts).

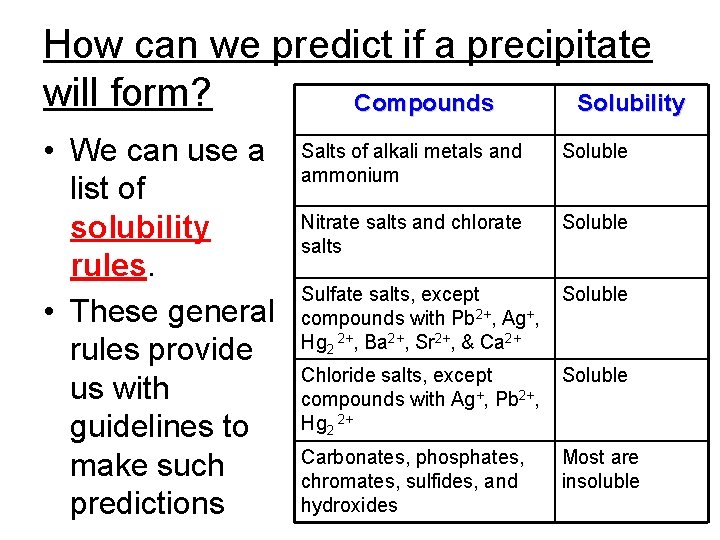
How can we predict if a precipitate will form? Compounds Solubility • We can use a list of solubility rules. • These general rules provide us with guidelines to make such predictions Salts of alkali metals and ammonium Soluble Nitrate salts and chlorate salts Soluble Sulfate salts, except compounds with Pb 2+, Ag+, Hg 2 2+, Ba 2+, Sr 2+, & Ca 2+ Soluble Chloride salts, except compounds with Ag+, Pb 2+, Hg 2 2+ Soluble Carbonates, phosphates, chromates, sulfides, and hydroxides Most are insoluble

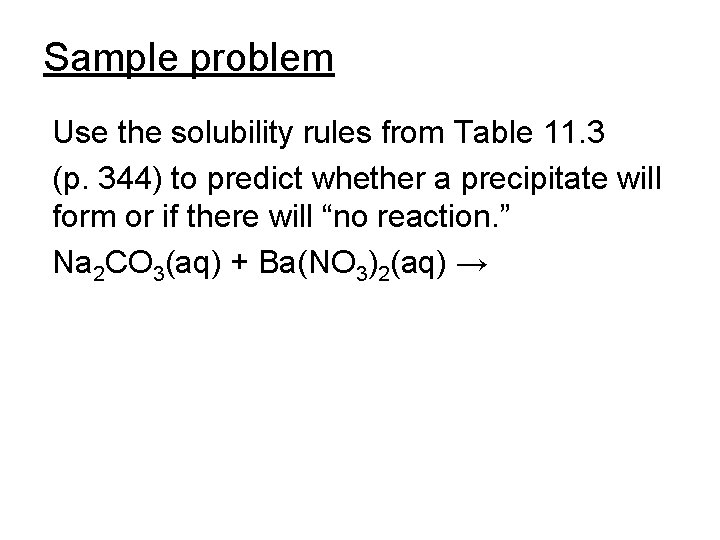
Sample problem Use the solubility rules from Table 11. 3 (p. 344) to predict whether a precipitate will form or if there will “no reaction. ” Na 2 CO 3(aq) + Ba(NO 3)2(aq) →

Chemical Reactions Chapter 11 The End