Chemical reactions can be classified Synthesis Reaction combines
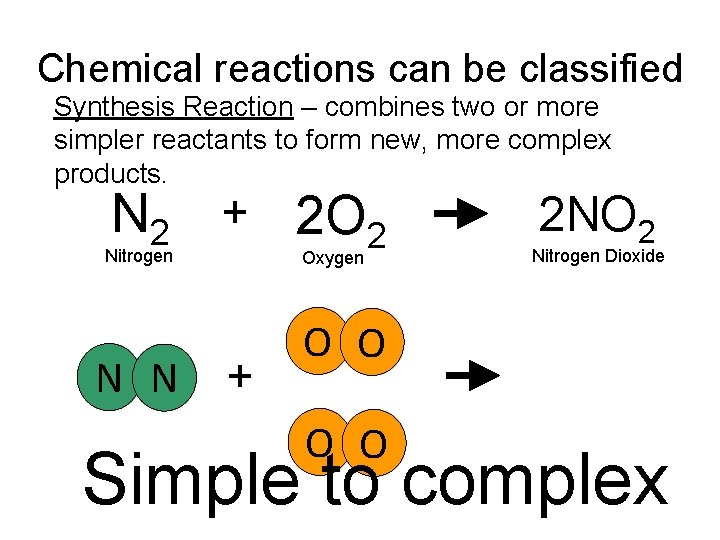
Chemical reactions can be classified Synthesis Reaction – combines two or more simpler reactants to form new, more complex products. N 2 + 2 O 2 Nitrogen N N Oxygen + 2 NO 2 Nitrogen Dioxide O O Simple to complex
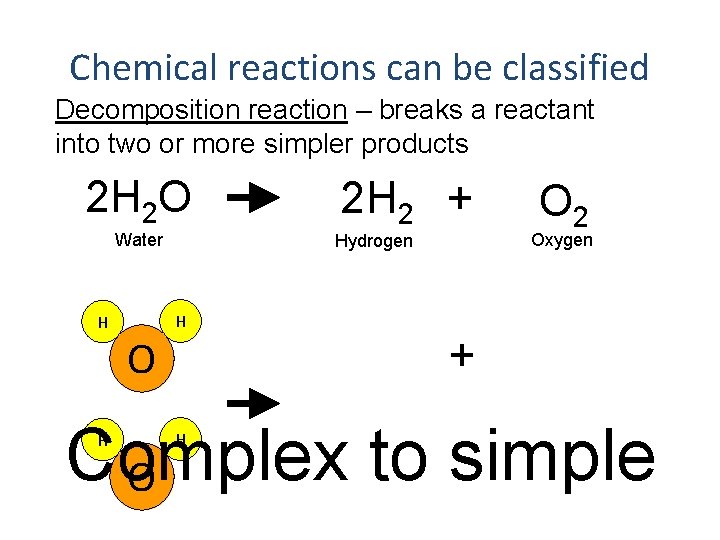
Chemical reactions can be classified Decomposition reaction – breaks a reactant into two or more simpler products 2 H 2 O Water H O 2 H 2 + Oxygen Hydrogen H O 2 + Complex to simple O H H
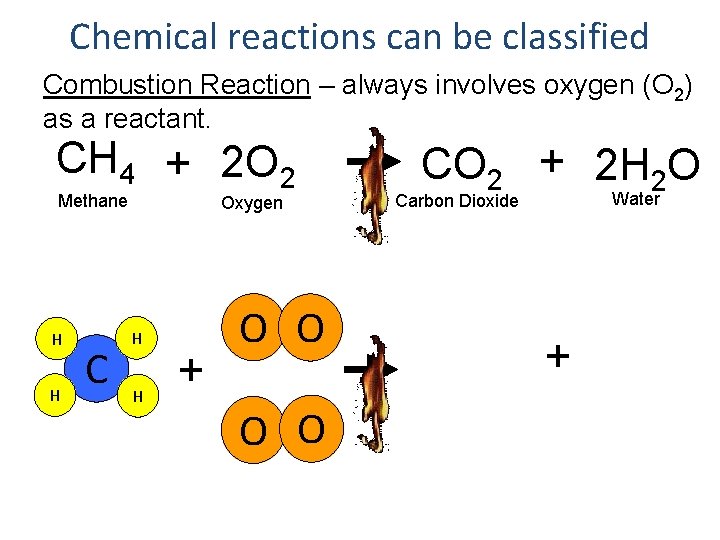
Chemical reactions can be classified Combustion Reaction – always involves oxygen (O 2) as a reactant. CH 4 + 2 O 2 Methane H H C Oxygen H H + O O CO 2 + 2 H 2 O Water Carbon Dioxide +
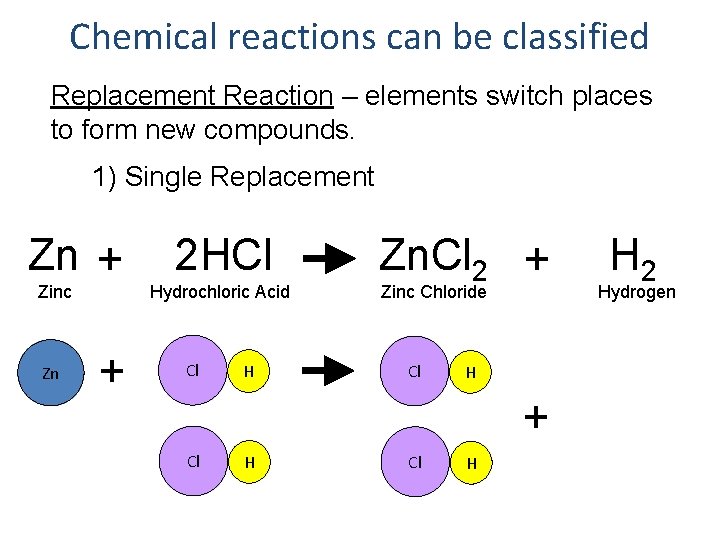
Chemical reactions can be classified Replacement Reaction – elements switch places to form new compounds. 1) Single Replacement Zn + Zinc Zn + 2 HCl Hydrochloric Acid Cl H Zn. Cl 2 + Zinc Chloride Cl H + Cl H H 2 Hydrogen
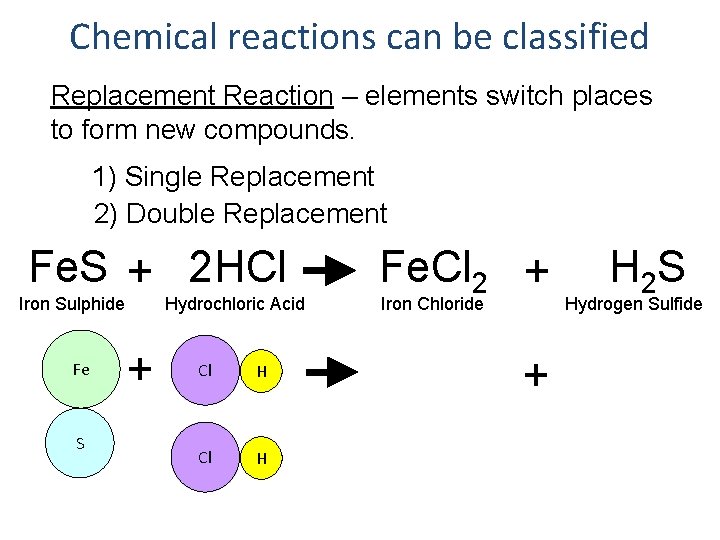
Chemical reactions can be classified Replacement Reaction – elements switch places to form new compounds. 1) Single Replacement 2) Double Replacement Fe. S + 2 HCl Iron Sulphide Fe S Hydrochloric Acid + Cl H Fe. Cl 2 + Iron Chloride + H 2 S Hydrogen Sulfide
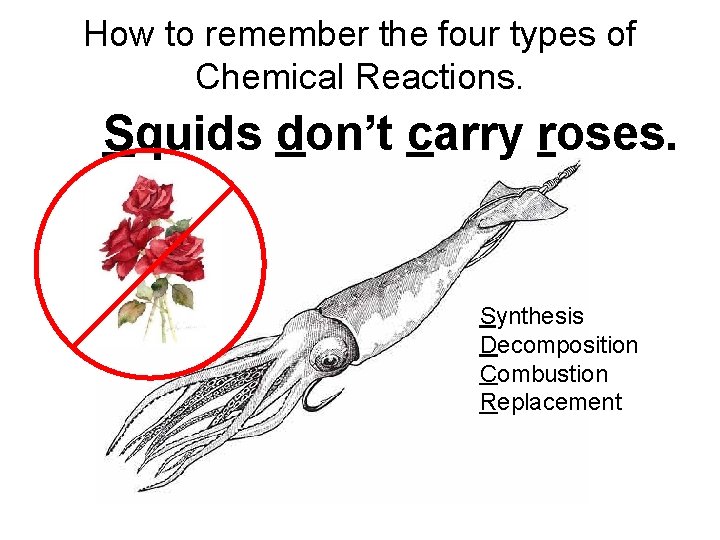
How to remember the four types of Chemical Reactions. Squids don’t carry roses. Synthesis Decomposition Combustion Replacement

Types of Chemical Reactions Sean Gillette, Ed. D Master Teacher Project Betterlesson. com July 13, 2014
- Slides: 7