CHEMICAL REACTIONS Balancing States of Matter and Writing

CHEMICAL REACTIONS Balancing, States of Matter, and Writing

Evidence for A Chemical Reaction o Five Signs of a Chemical Change 1. 2. 3. 4. 5. Gas Given Off (Bubbles) Color Change (BIGGIE!!!!!!) Heat is Produced or Absorbed Odor Change Precipitate Forms (Solid is Made)
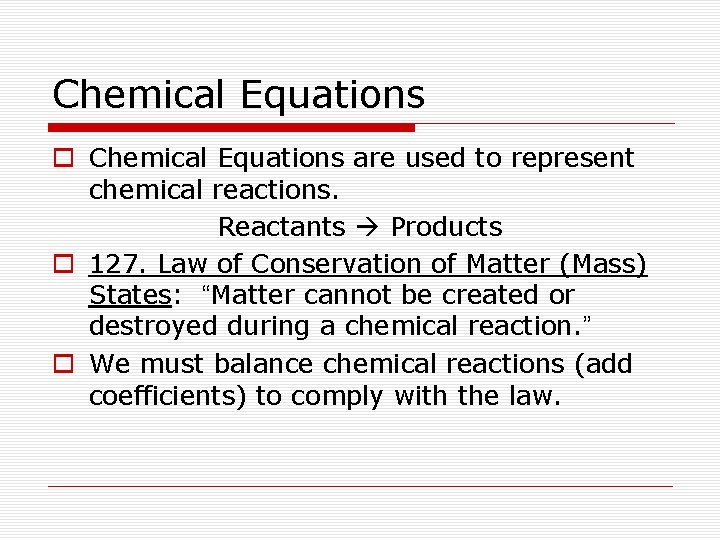
Chemical Equations o Chemical Equations are used to represent chemical reactions. Reactants Products o 127. Law of Conservation of Matter (Mass) States: “Matter cannot be created or destroyed during a chemical reaction. ” o We must balance chemical reactions (add coefficients) to comply with the law.
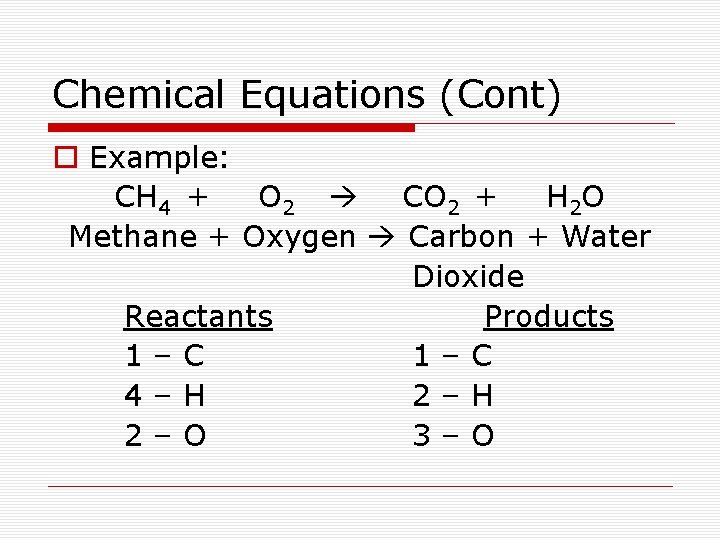
Chemical Equations (Cont) o Example: CH 4 + O 2 CO 2 + H 2 O Methane + Oxygen Carbon + Water Dioxide Reactants Products 1–C 4–H 2–O 3–O

Balancing o Chemical reactions change the groupings of the atoms, but DO NOT create or destroy atoms, so there must be the same number of each type of atom on each side of the arrow. We must use coefficients to Balance a Chemical Equation

Balancing Chemical Equations 1. Determine the correct formula for each reactant and product by supplying the subscripts to the formulas. 1. Once you write the correct formula, DO NOT change the subscripts. 2. Add coefficients in front of balanced formulas to give the same number and kind of atoms on both sides. 1. Use the lowest possible ratio of coefficients.
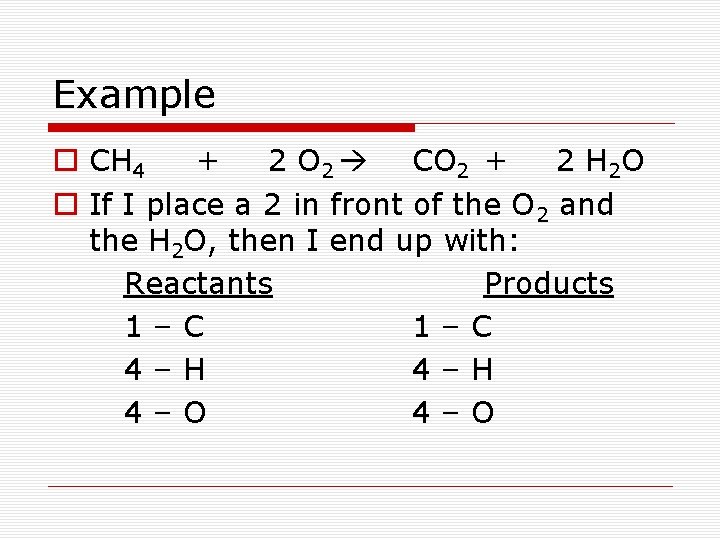
Example o CH 4 + 2 O 2 CO 2 + 2 H 2 O o If I place a 2 in front of the O 2 and the H 2 O, then I end up with: Reactants Products 1–C 4–H 4–O
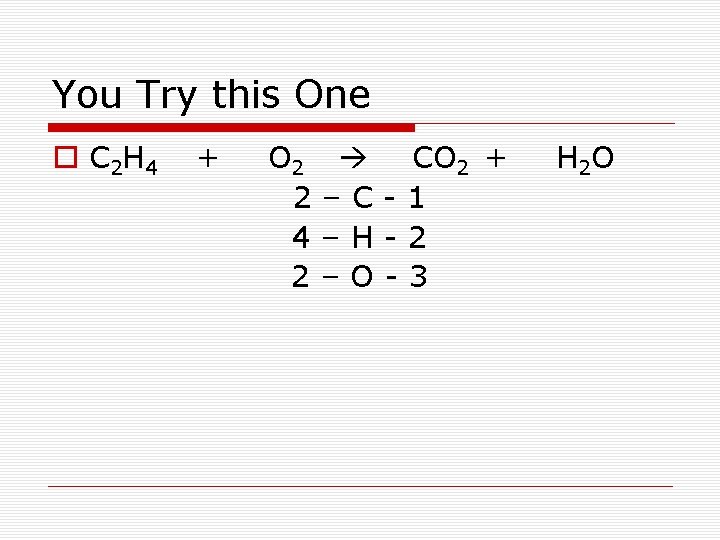
You Try this One o C 2 H 4 + O 2 2 4 2 CO 2 + –C-1 –H-2 –O-3 H 2 O
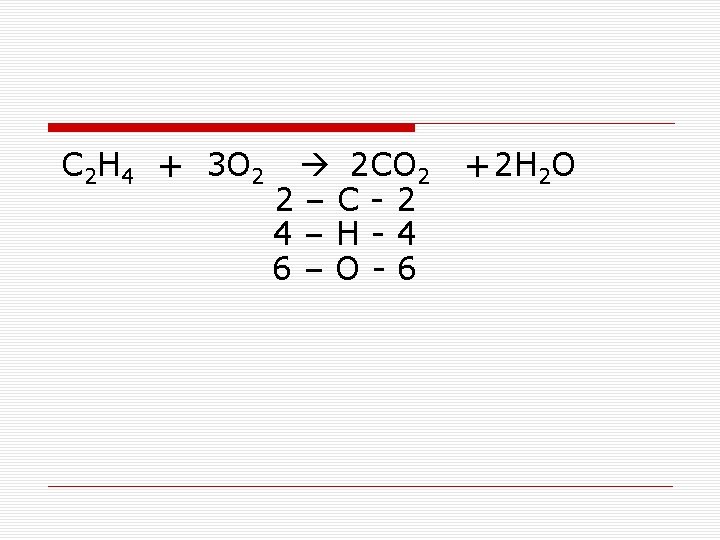
C 2 H 4 + 3 O 2 2 CO 2 2–C-2 4–H-4 6–O-6 +2 H 2 O
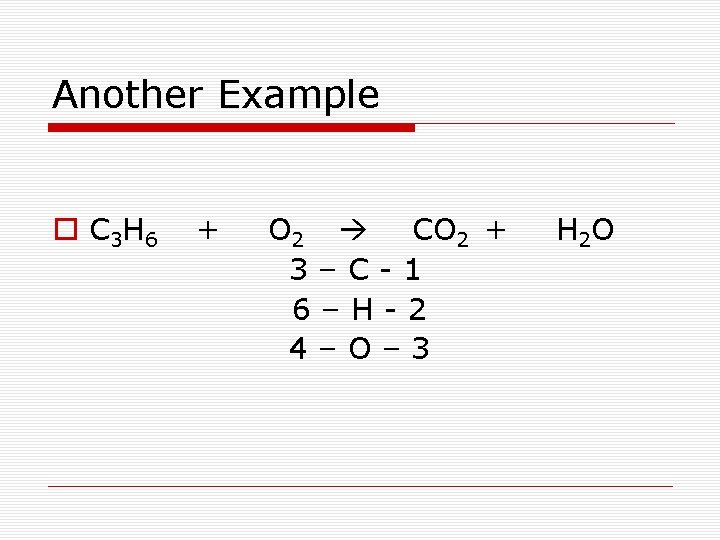
Another Example o C 3 H 6 + O 2 CO 2 + 3–C-1 6–H-2 4–O– 3 H 2 O
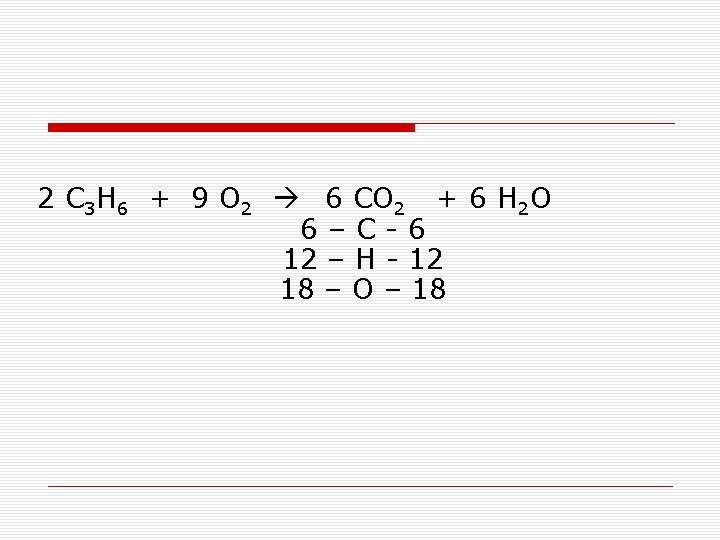
2 C 3 H 6 + 9 O 2 6 CO 2 + 6 H 2 O 6–C-6 12 – H - 12 18 – O – 18
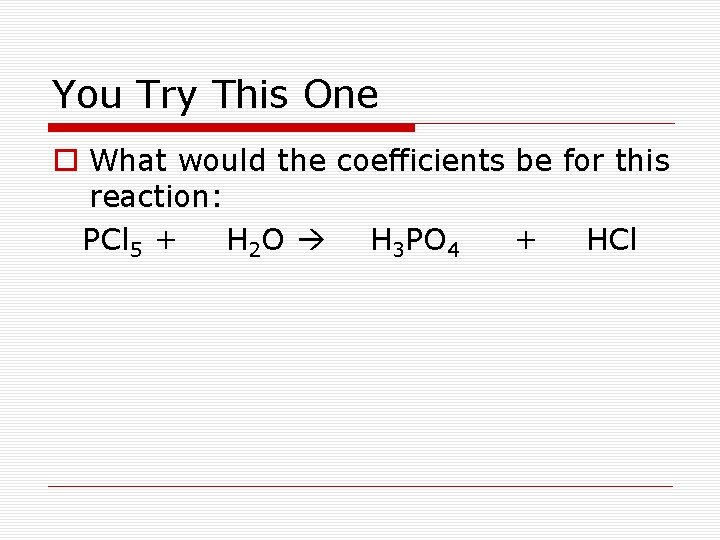
You Try This One o What would the coefficients be for this reaction: PCl 5 + H 2 O H 3 PO 4 + HCl
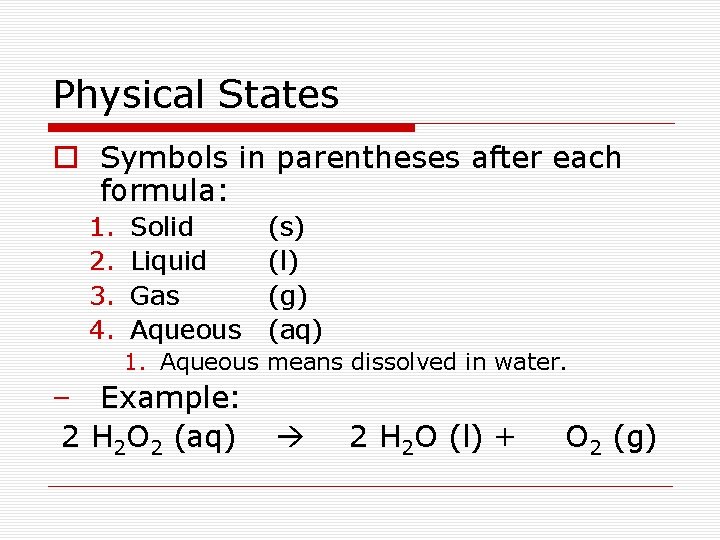
Physical States o Symbols in parentheses after each formula: 1. 2. 3. 4. Solid Liquid Gas Aqueous (s) (l) (g) (aq) 1. Aqueous means dissolved in water. – Example: 2 H 2 O 2 (aq) 2 H 2 O (l) + O 2 (g)

Words to watch for o States of matter – This is a symbol that is used to identify the state of matter for each substance in a reaction. o Gas, Liquid, Solid, Aqueous n Gas (g) n Liquid (l) n Solid (s) may also be called a Precipitant (ppt) or a crystal (c) n Aqueous (aq) means dissolved in water.
- Slides: 14