Chemical Reactions Balancing Chemical Equations Types of Chemical

Chemical Reactions • Balancing Chemical Equations • Types of Chemical Reactions • Synthesis Reactions • Decomposition Reactions • Single-Replacement Reactions • • Combustion Reactions Double-Replacement Reactions • Oxidation-Reduction Reactions
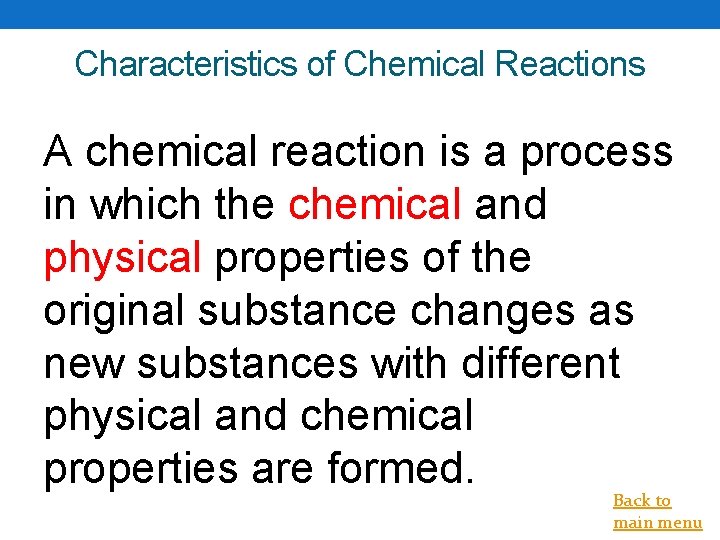
Characteristics of Chemical Reactions A chemical reaction is a process in which the chemical and physical properties of the original substance changes as new substances with different physical and chemical properties are formed. Back to main menu
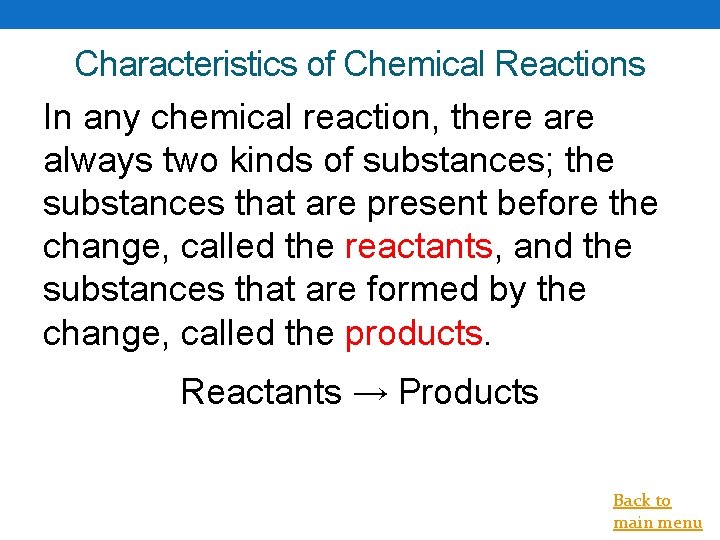
Characteristics of Chemical Reactions In any chemical reaction, there always two kinds of substances; the substances that are present before the change, called the reactants, and the substances that are formed by the change, called the products. Reactants → Products Back to main menu
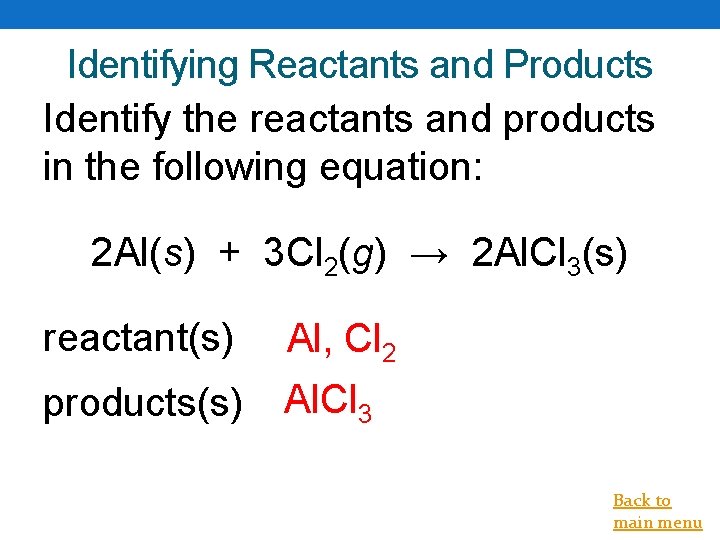
Identifying Reactants and Products Identify the reactants and products in the following equation: 2 Al(s) + 3 Cl 2(g) → 2 Al. Cl 3(s) reactant(s) Al, Cl 2 products(s) Al. Cl 3 Back to main menu
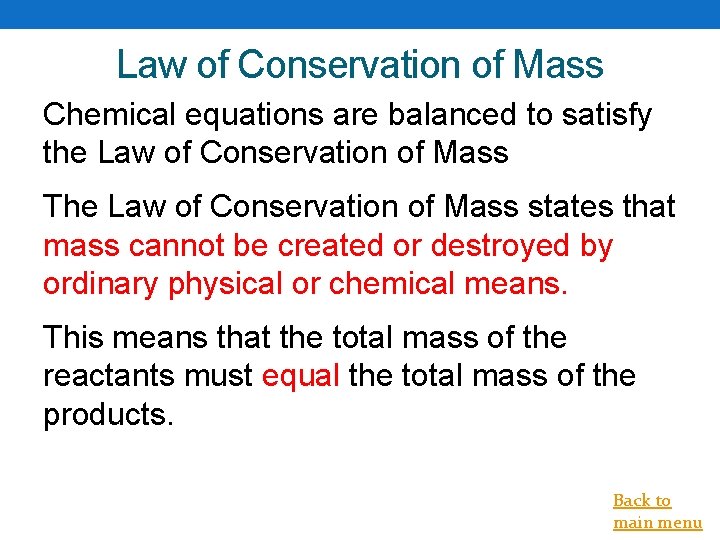
Law of Conservation of Mass Chemical equations are balanced to satisfy the Law of Conservation of Mass The Law of Conservation of Mass states that mass cannot be created or destroyed by ordinary physical or chemical means. This means that the total mass of the reactants must equal the total mass of the products. Back to main menu
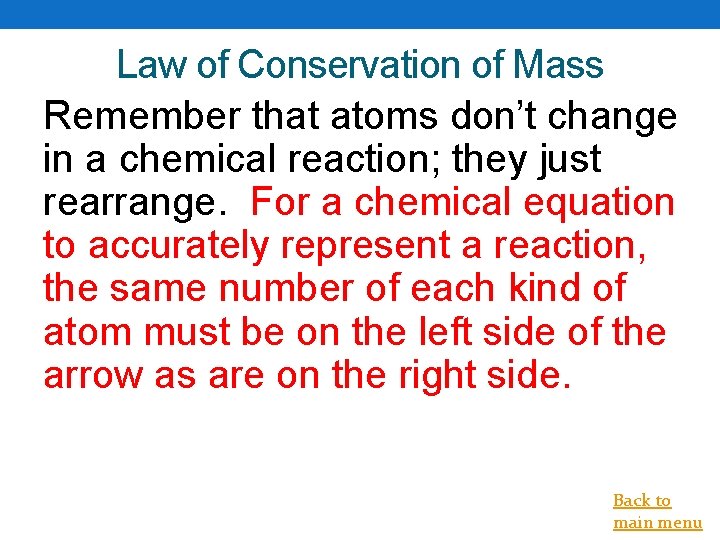
Law of Conservation of Mass Remember that atoms don’t change in a chemical reaction; they just rearrange. For a chemical equation to accurately represent a reaction, the same number of each kind of atom must be on the left side of the arrow as are on the right side. Back to main menu
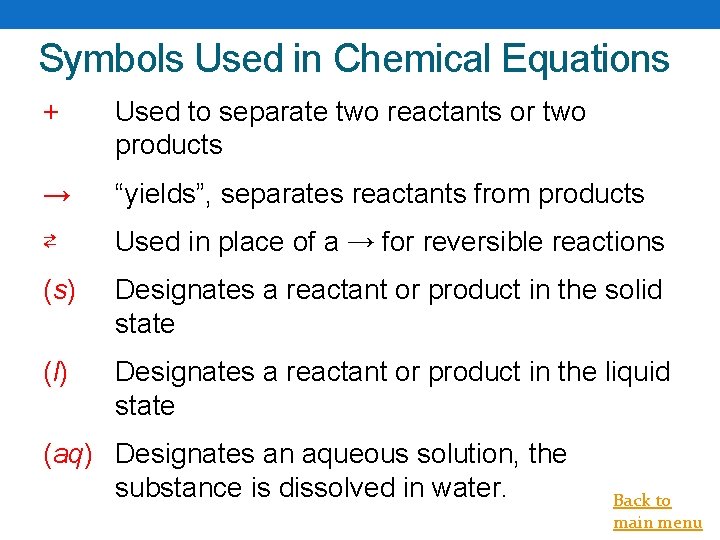
Symbols Used in Chemical Equations + Used to separate two reactants or two products → “yields”, separates reactants from products ⇄ Used in place of a → for reversible reactions (s) Designates a reactant or product in the solid state (l) Designates a reactant or product in the liquid state (aq) Designates an aqueous solution, the substance is dissolved in water. Back to main menu
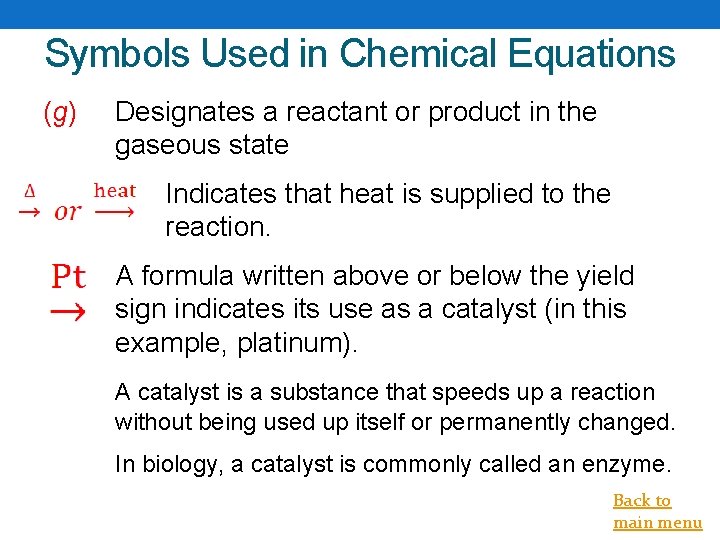
Symbols Used in Chemical Equations (g) Designates a reactant or product in the gaseous state Indicates that heat is supplied to the reaction. A formula written above or below the yield sign indicates its use as a catalyst (in this example, platinum). A catalyst is a substance that speeds up a reaction without being used up itself or permanently changed. In biology, a catalyst is commonly called an enzyme. Back to main menu
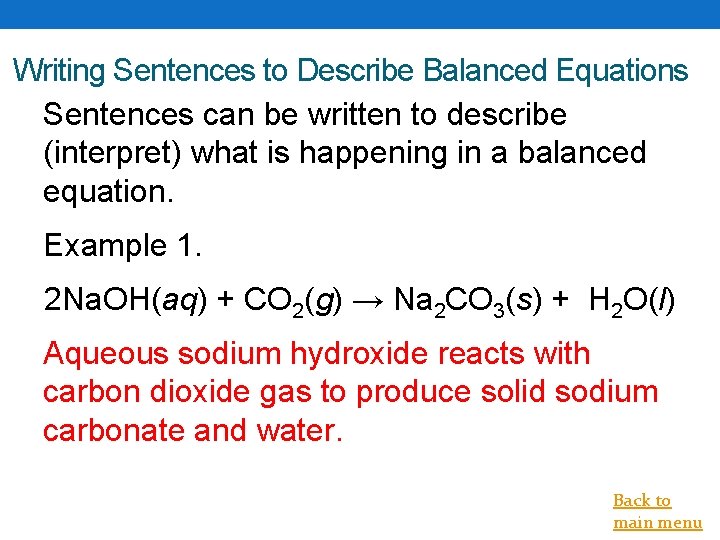
Writing Sentences to Describe Balanced Equations Sentences can be written to describe (interpret) what is happening in a balanced equation. Example 1. 2 Na. OH(aq) + CO 2(g) → Na 2 CO 3(s) + H 2 O(l) Aqueous sodium hydroxide reacts with carbon dioxide gas to produce solid sodium carbonate and water. Back to main menu
Writing Sentences to Describe Balanced Equations Example 2. 2 Al(s) + 3 Cl 2(g) → 2 Al. Cl 3(s) Aluminum metal reacts with chlorine gas to produce solid aluminum chloride. Back to main menu
You Try It Write sentences to describe each of the following equations. (Do not worry about the coefficients at this time. ) 3. 2 H 2(g) + O 2(g) → 2 H 2 O(l) Hydrogen gas reacts with oxygen gas to produce water. 4. Zn(s) + Cu. Cl 2(aq) → Zn. Cl 2(aq) + Cu(s) Zinc metal reacts with aqueous copper(II) chloride to produce aqueous zinc chloride and copper metal. Back to main menu
Indications of a Chemical Reaction What are the four observations that generally indicate that a chemical reaction has occurred? 1. Production of a gas. 2. Formation of a precipitate. (an insoluble solid that forms in an aqueous reaction) 3. Change in energy. Endothermic reaction – energy is absorbed Exothermic Reaction – energy is released 4. Change in color or odor. Back to main menu
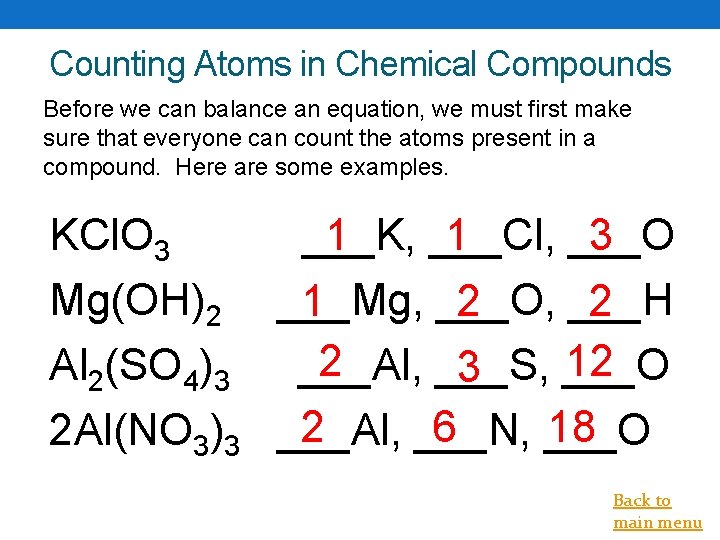
Counting Atoms in Chemical Compounds Before we can balance an equation, we must first make sure that everyone can count the atoms present in a compound. Here are some examples. 1 KCl. O 3 ___K, ___Cl, 1 ___O 3 Mg(OH)2 ___Mg, ___O, ___H 1 2 2 2 12 Al 2(SO 4)3 ___Al, ___S, ___O 3 2 6 18 2 Al(NO 3)3 ___Al, ___N, ___O Back to main menu
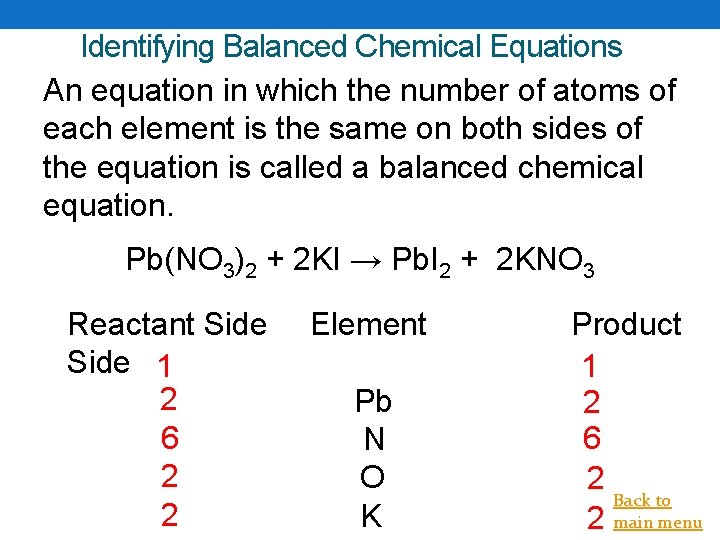
Identifying Balanced Chemical Equations An equation in which the number of atoms of each element is the same on both sides of the equation is called a balanced chemical equation. Pb(NO 3)2 + 2 KI → Pb. I 2 + 2 KNO 3 Reactant Side 1 2 6 2 2 Element Pb N O K Product 1 2 6 2 Back to 2 main menu
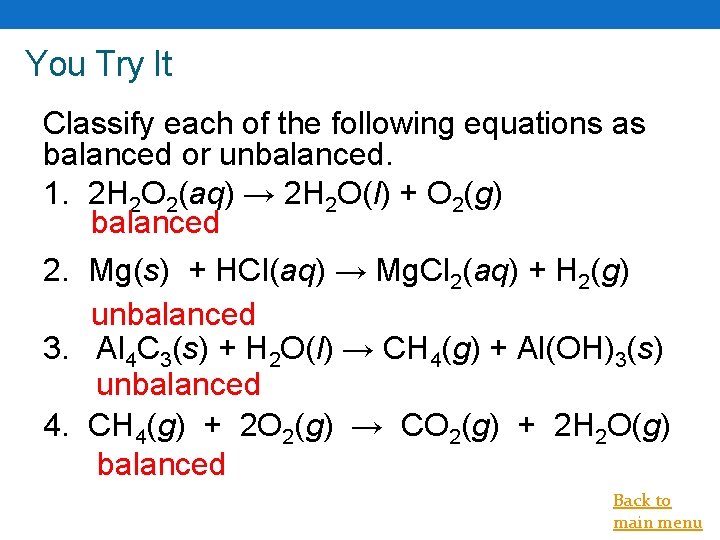
You Try It Classify each of the following equations as balanced or unbalanced. 1. 2 H 2 O 2(aq) → 2 H 2 O(l) + O 2(g) balanced 2. Mg(s) + HCl(aq) → Mg. Cl 2(aq) + H 2(g) unbalanced 3. Al 4 C 3(s) + H 2 O(l) → CH 4(g) + Al(OH)3(s) unbalanced 4. CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) balanced Back to main menu
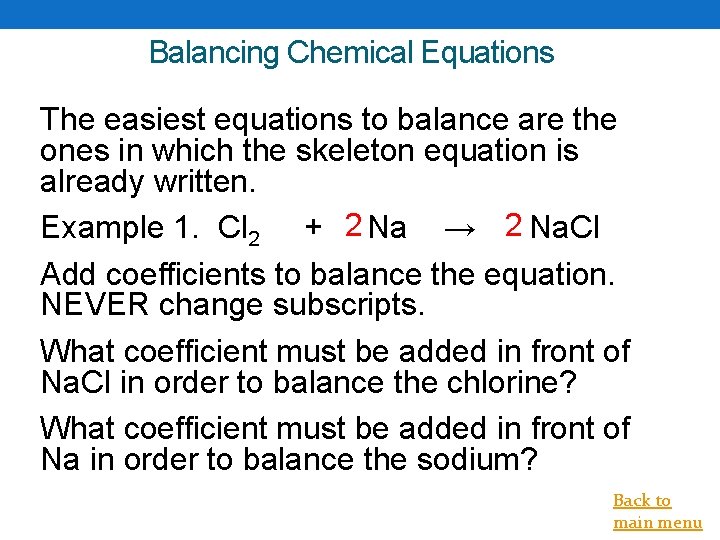
Balancing Chemical Equations The easiest equations to balance are the ones in which the skeleton equation is already written. Example 1. Cl 2 + 2 Na → 2 Na. Cl Add coefficients to balance the equation. NEVER change subscripts. What coefficient must be added in front of Na. Cl in order to balance the chlorine? What coefficient must be added in front of Na in order to balance the sodium? Back to main menu
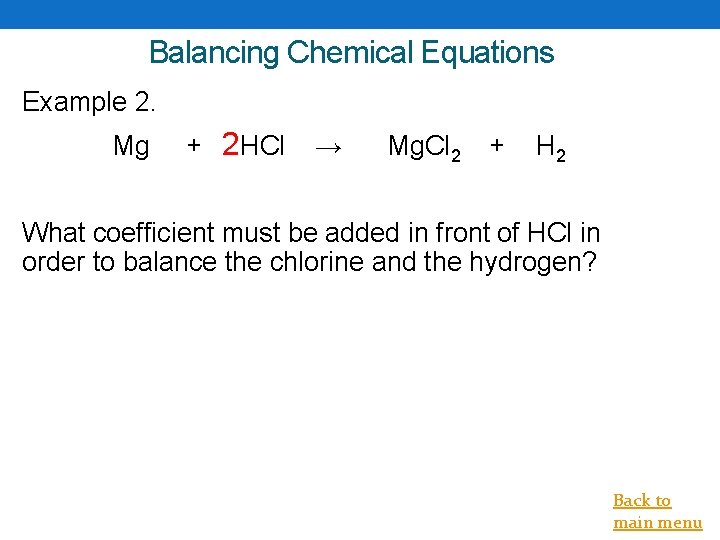
Balancing Chemical Equations Example 2. Mg + 2 HCl → Mg. Cl 2 + H 2 What coefficient must be added in front of HCl in order to balance the chlorine and the hydrogen? Back to main menu
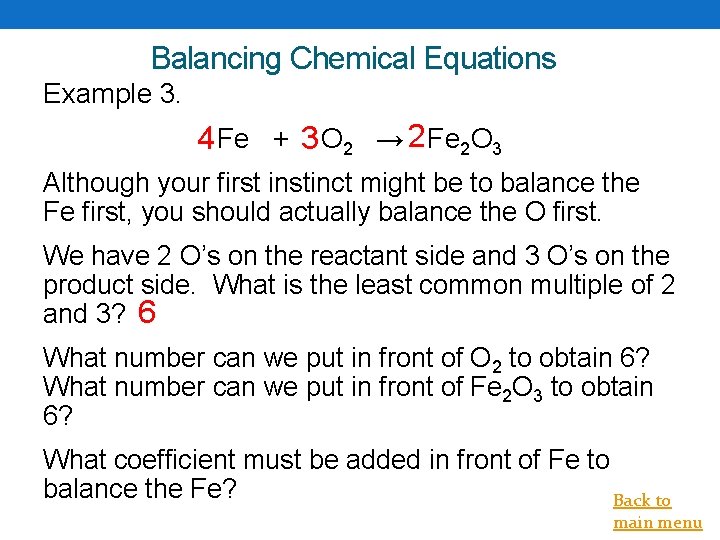
Balancing Chemical Equations Example 3. 4 Fe + 3 O 2 → 2 Fe 2 O 3 Although your first instinct might be to balance the Fe first, you should actually balance the O first. We have 2 O’s on the reactant side and 3 O’s on the product side. What is the least common multiple of 2 and 3? 6 What number can we put in front of O 2 to obtain 6? What number can we put in front of Fe 2 O 3 to obtain 6? What coefficient must be added in front of Fe to balance the Fe? Back to main menu
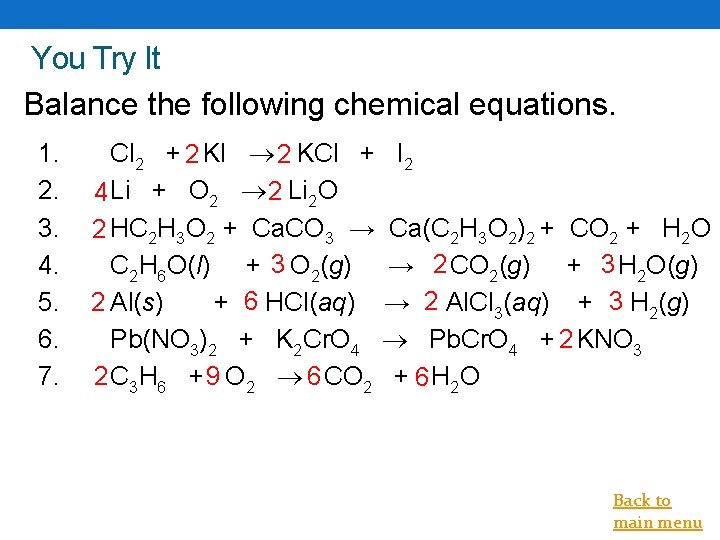
You Try It Balance the following chemical equations. 1. 2. 3. 4. 5. 6. 7. Cl 2 + 2 KI 2 KCl + I 2 4 Li + O 2 2 Li 2 O 2 HC 2 H 3 O 2 + Ca. CO 3 → Ca(C 2 H 3 O 2)2 + CO 2 + H 2 O C 2 H 6 O(l) + 3 O 2(g) → 2 CO 2(g) + 3 H 2 O(g) 2 Al(s) + 6 HCl(aq) → 2 Al. Cl 3(aq) + 3 H 2(g) Pb(NO 3)2 + K 2 Cr. O 4 Pb. Cr. O 4 + 2 KNO 3 2 C 3 H 6 + 9 O 2 6 CO 2 + 6 H 2 O Back to main menu
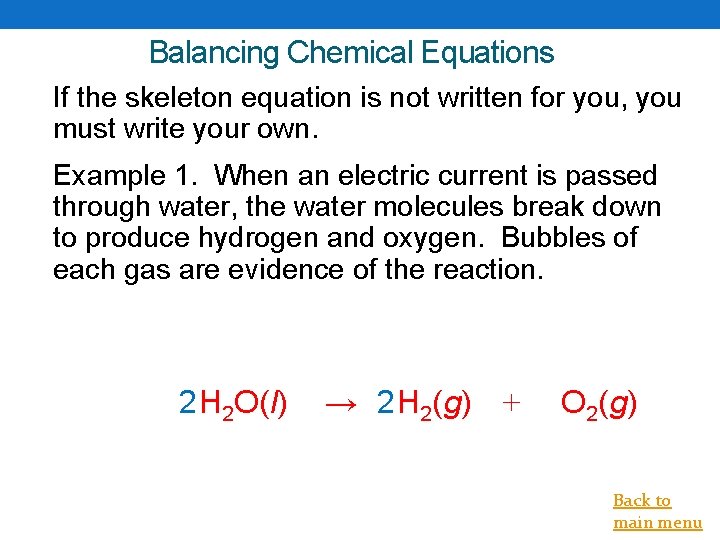
Balancing Chemical Equations If the skeleton equation is not written for you, you must write your own. Example 1. When an electric current is passed through water, the water molecules break down to produce hydrogen and oxygen. Bubbles of each gas are evidence of the reaction. 2 H 2 O(l) → 2 H 2(g) + O 2(g) Back to main menu
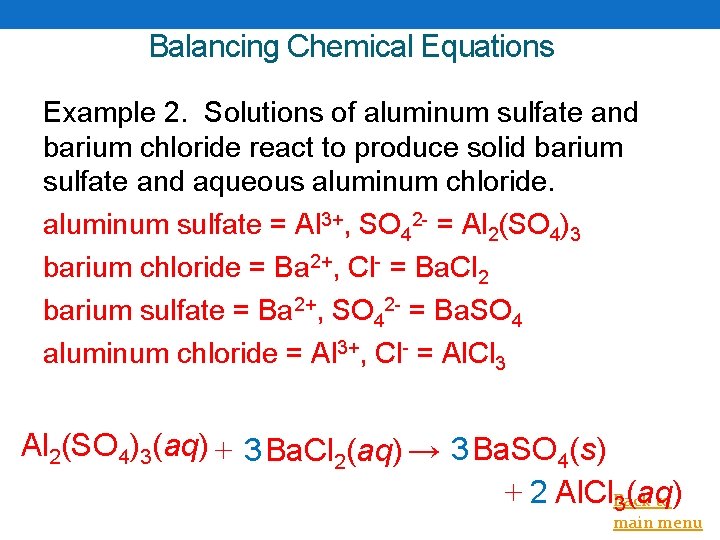
Balancing Chemical Equations Example 2. Solutions of aluminum sulfate and barium chloride react to produce solid barium sulfate and aqueous aluminum chloride. aluminum sulfate = Al 3+, SO 42 - = Al 2(SO 4)3 barium chloride = Ba 2+, Cl- = Ba. Cl 2 barium sulfate = Ba 2+, SO 42 - = Ba. SO 4 aluminum chloride = Al 3+, Cl- = Al. Cl 3 Al 2(SO 4)3(aq) + 3 Ba. Cl 2(aq) → 3 Ba. SO 4(s) + 2 Al. Cl. Back to 3(aq) main menu
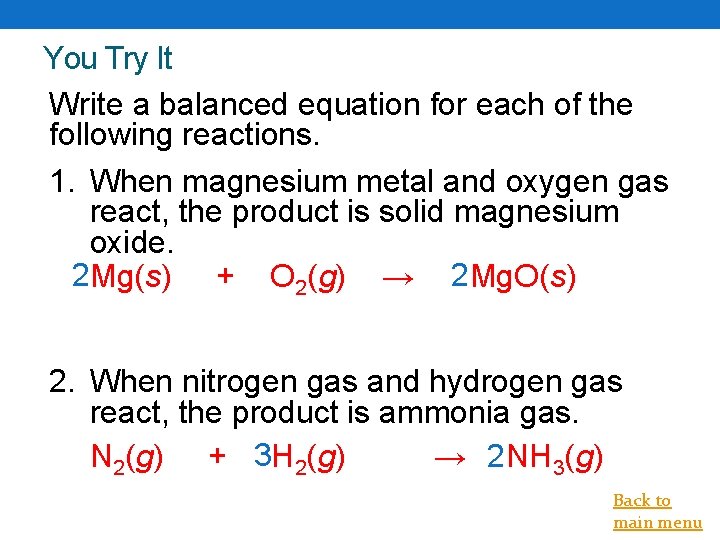
You Try It Write a balanced equation for each of the following reactions. 1. When magnesium metal and oxygen gas react, the product is solid magnesium oxide. 2 Mg(s) + O 2(g) → 2 Mg. O(s) 2. When nitrogen gas and hydrogen gas react, the product is ammonia gas. N 2(g) + 3 H 2(g) → 2 NH 3(g) Back to main menu
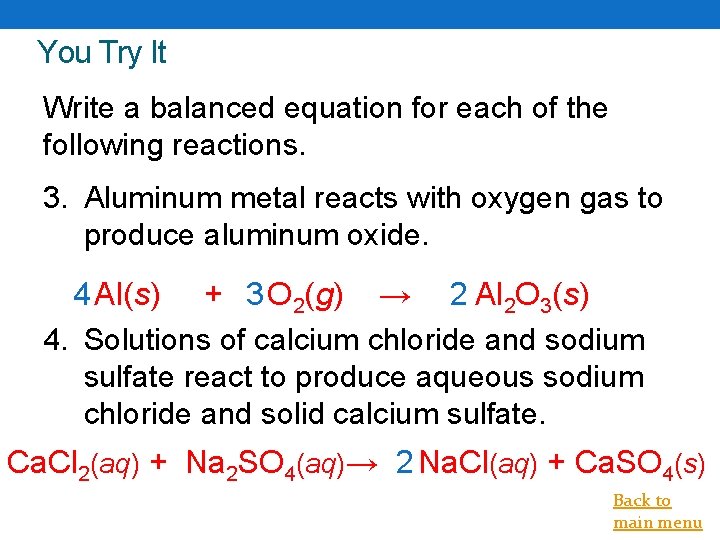
You Try It Write a balanced equation for each of the following reactions. 3. Aluminum metal reacts with oxygen gas to produce aluminum oxide. 4 Al(s) + 3 O 2(g) → 2 Al 2 O 3(s) 4. Solutions of calcium chloride and sodium sulfate react to produce aqueous sodium chloride and solid calcium sulfate. Ca. Cl 2(aq) + Na 2 SO 4(aq)→ 2 Na. Cl(aq) + Ca. SO 4(s) Back to main menu
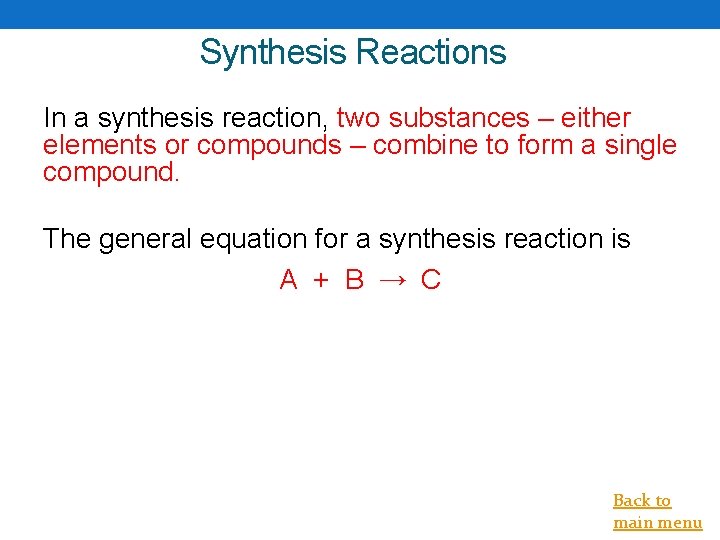
Synthesis Reactions In a synthesis reaction, two substances – either elements or compounds – combine to form a single compound. The general equation for a synthesis reaction is A + B → C Back to main menu
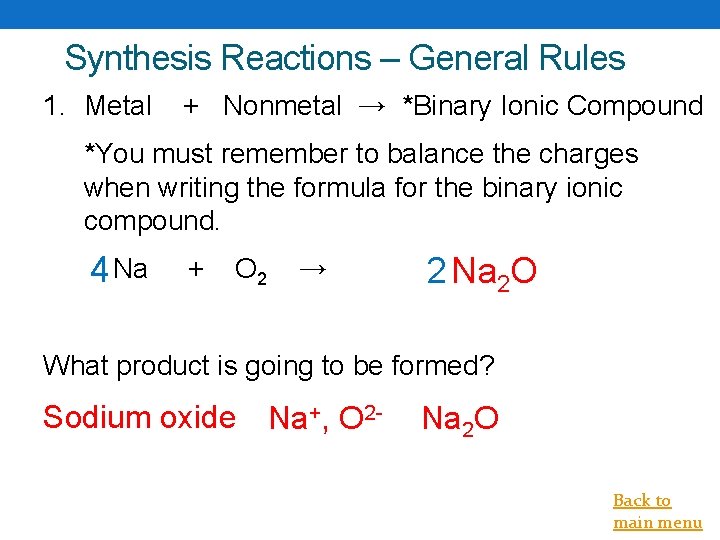
Synthesis Reactions – General Rules 1. Metal + Nonmetal → *Binary Ionic Compound *You must remember to balance the charges when writing the formula for the binary ionic compound. 4 Na + O 2 → 2 Na 2 O What product is going to be formed? Sodium oxide Na+, O 2 - Na 2 O Back to main menu
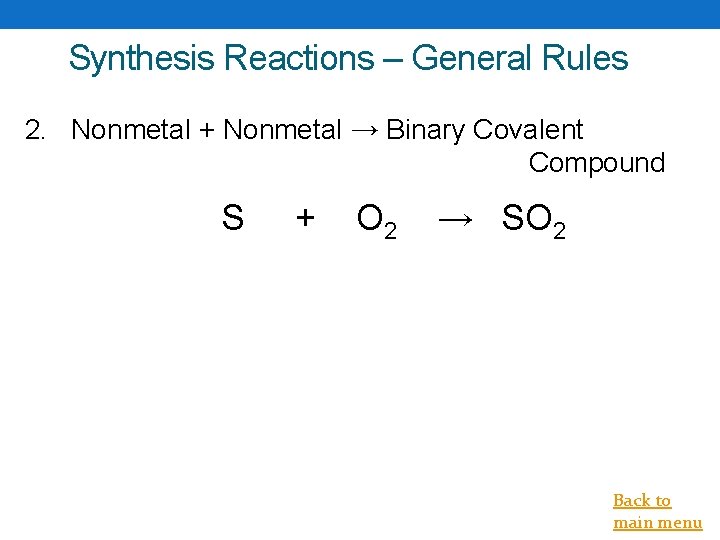
Synthesis Reactions – General Rules 2. Nonmetal + Nonmetal → Binary Covalent Compound S + O 2 → SO 2 Back to main menu
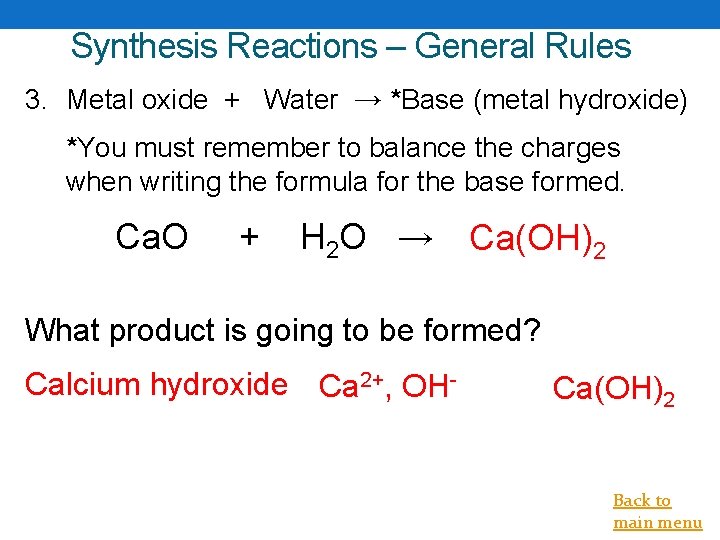
Synthesis Reactions – General Rules 3. Metal oxide + Water → *Base (metal hydroxide) *You must remember to balance the charges when writing the formula for the base formed. Ca. O + H 2 O → Ca(OH)2 What product is going to be formed? Calcium hydroxide Ca 2+, OH- Ca(OH)2 Back to main menu
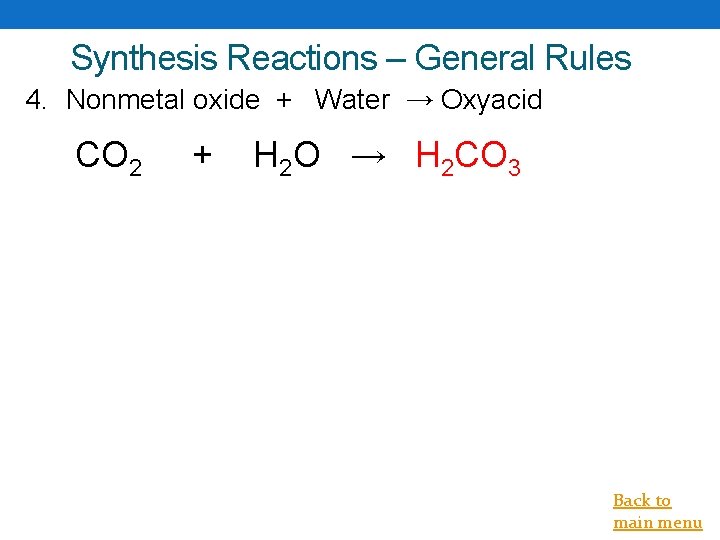
Synthesis Reactions – General Rules 4. Nonmetal oxide + Water → Oxyacid CO 2 + H 2 O → H 2 CO 3 Back to main menu
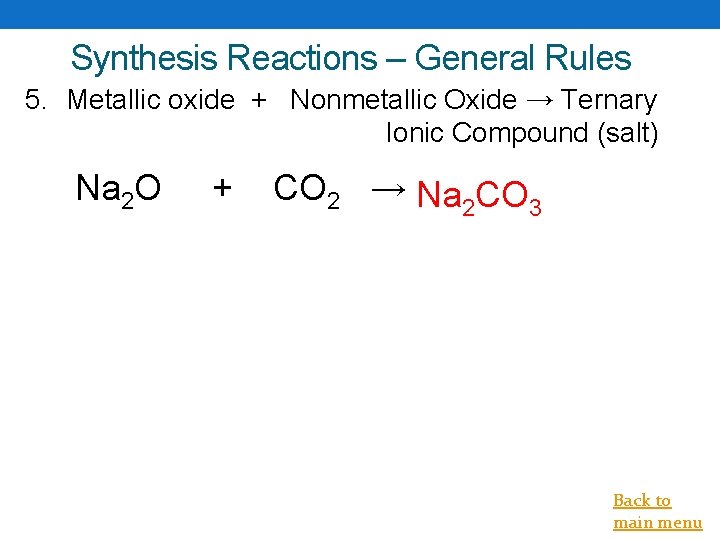
Synthesis Reactions – General Rules 5. Metallic oxide + Nonmetallic Oxide → Ternary Ionic Compound (salt) Na 2 O + CO 2 → Na 2 CO 3 Back to main menu
Synthesis Reactions – General Rules 6. Here are two synthesis reactions that must be memorized. N 2(g) + 3 H 2(g) → 2 NH 3(g) + H 2 O(l) → NH 4 OH(aq) Back to main menu
You Try It Balance the following equations. You may need to complete the equation before balancing it. 1. 4 Li + O 2 → 2 Li 2 O 2. 2 Mg + O 2 → 2 Mg. O 3. Mg + Cl 2 → Mg. Cl 2 4. Ca. O + SO 3 → Ca. SO 4 5. SO 2 + H 2 O → H 2 SO 3 6. Na 2 O + H 2 O → 2 Na. OH Back to main menu
You Try It Balance the following equations. You may need to complete the equation before balancing it. Ca 3 N 2 1. 3 Ca + N 2 → 2. P 4 O 10 + 6 H 2 O → 4 H 3 PO 4 3. I 2 + 3 Cl 2 → 2 ICl 3 4. Sr. O + H 2 O → Sr(OH)2 Back to main menu
Decomposition Reactions In a decomposition reaction, a compound breaks down into two or more simpler substances. The compound may break down into individual elements, a compound an element, or into simpler compounds. Many decomposition reaction take place only when energy is added in the form of heat or light. Electrolysis is the decomposition of a substance by an electric current. The general equation for a decomposition reaction is C→ A+B Back to main menu
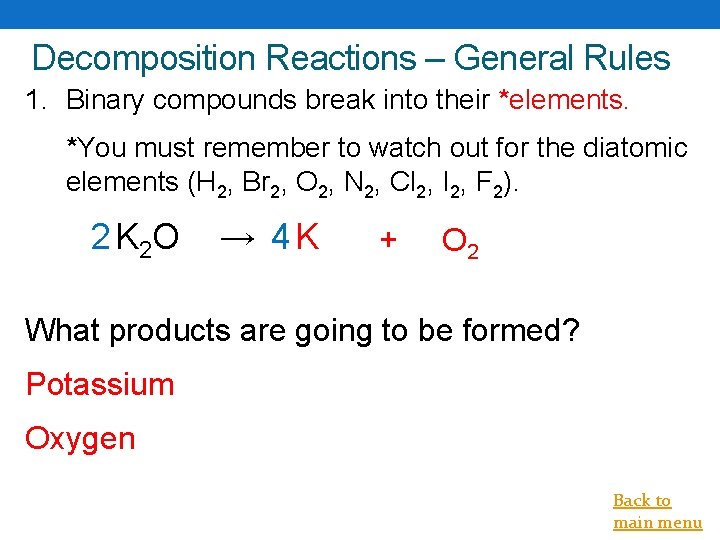
Decomposition Reactions – General Rules 1. Binary compounds break into their *elements. *You must remember to watch out for the diatomic elements (H 2, Br 2, O 2, N 2, Cl 2, I 2, F 2). 2 K 2 O → 4 K + O 2 What products are going to be formed? Potassium Oxygen Back to main menu
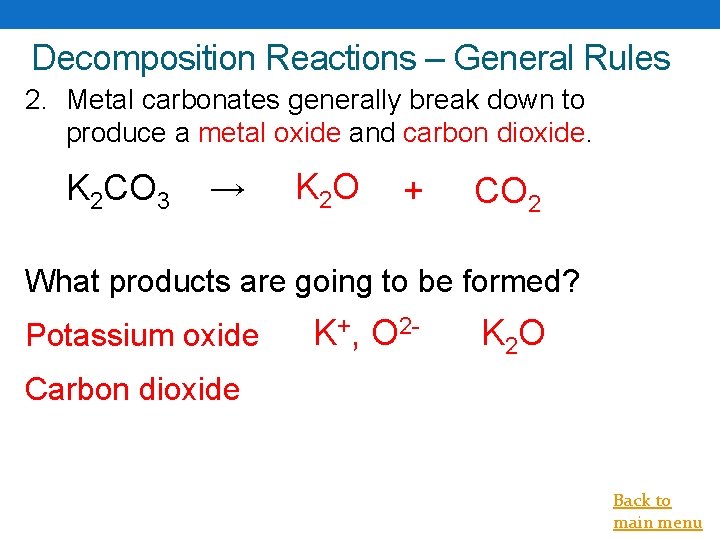
Decomposition Reactions – General Rules 2. Metal carbonates generally break down to produce a metal oxide and carbon dioxide. K 2 CO 3 → K 2 O + CO 2 What products are going to be formed? Potassium oxide K+, O 2 - K 2 O Carbon dioxide Back to main menu
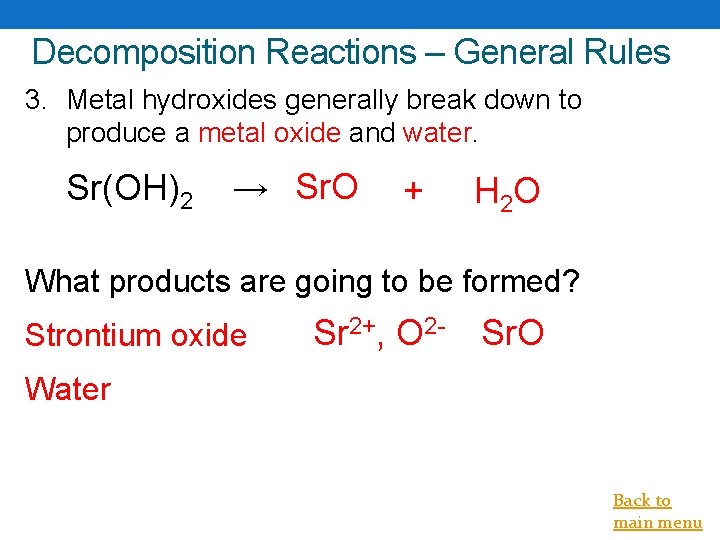
Decomposition Reactions – General Rules 3. Metal hydroxides generally break down to produce a metal oxide and water. Sr(OH)2 → Sr. O + H 2 O What products are going to be formed? Strontium oxide Sr 2+, O 2 - Sr. O Water Back to main menu
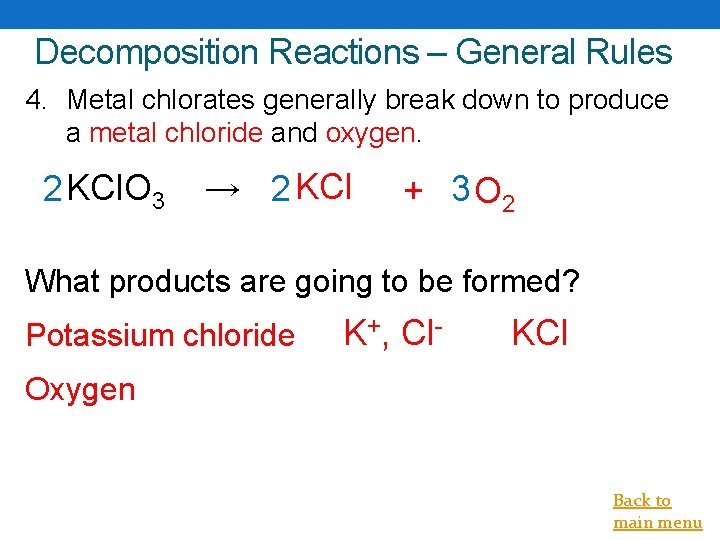
Decomposition Reactions – General Rules 4. Metal chlorates generally break down to produce a metal chloride and oxygen. 2 KCl. O 3 → 2 KCl + 3 O 2 What products are going to be formed? Potassium chloride K+, Cl- KCl Oxygen Back to main menu
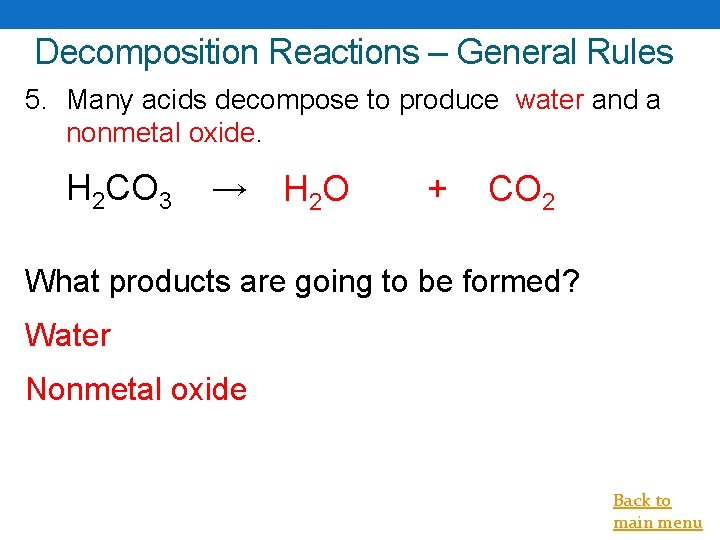
Decomposition Reactions – General Rules 5. Many acids decompose to produce water and a nonmetal oxide. H 2 CO 3 → H 2 O + CO 2 What products are going to be formed? Water Nonmetal oxide Back to main menu
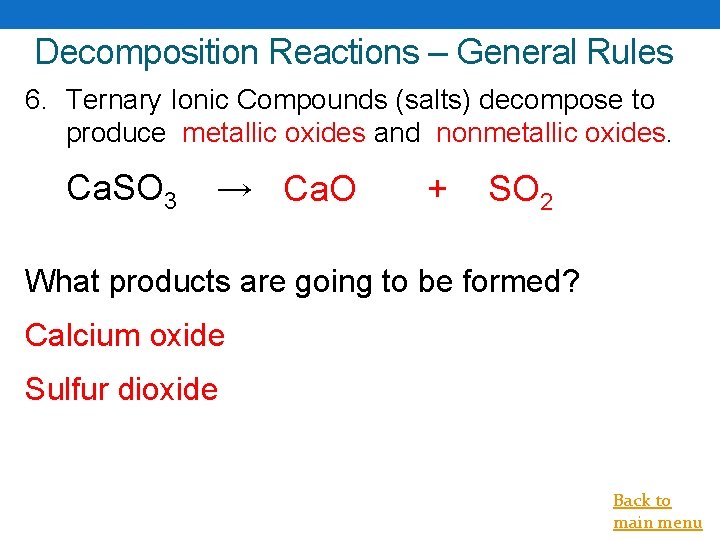
Decomposition Reactions – General Rules 6. Ternary Ionic Compounds (salts) decompose to produce metallic oxides and nonmetallic oxides. Ca. SO 3 → Ca. O + SO 2 What products are going to be formed? Calcium oxide Sulfur dioxide Back to main menu
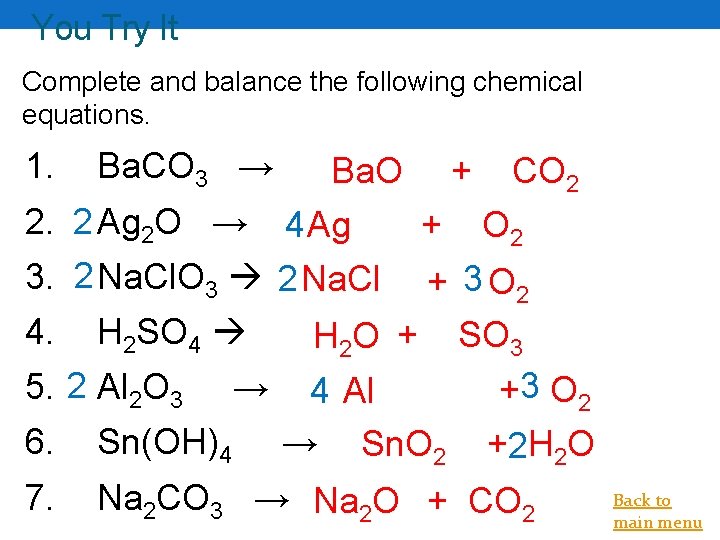
You Try It Complete and balance the following chemical equations. 1. 2. 3. 4. 5. Ba. CO 3 → Ba. O + CO 2 2 Ag 2 O → 4 Ag + O 2 2 Na. Cl. O 3 2 Na. Cl + 3 O 2 H 2 SO 4 H 2 O + SO 3 2 Al 2 O 3 → 4 Al +3 O 2 6. Sn(OH)4 → 7. Na 2 CO 3 → Na 2 O + CO 2 Sn. O 2 +2 H 2 O Back to main menu
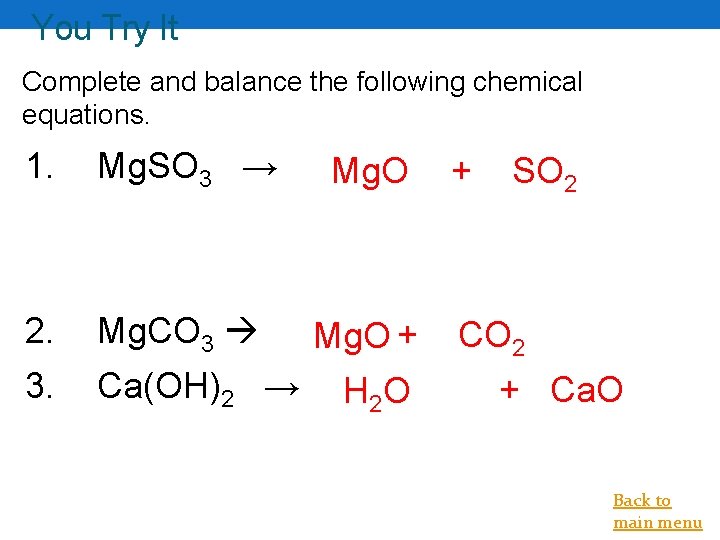
You Try It Complete and balance the following chemical equations. 1. Mg. SO 3 → Mg. O 2. Mg. CO 3 Mg. O + 3. Ca(OH)2 → H 2 O + SO 2 CO 2 + Ca. O Back to main menu
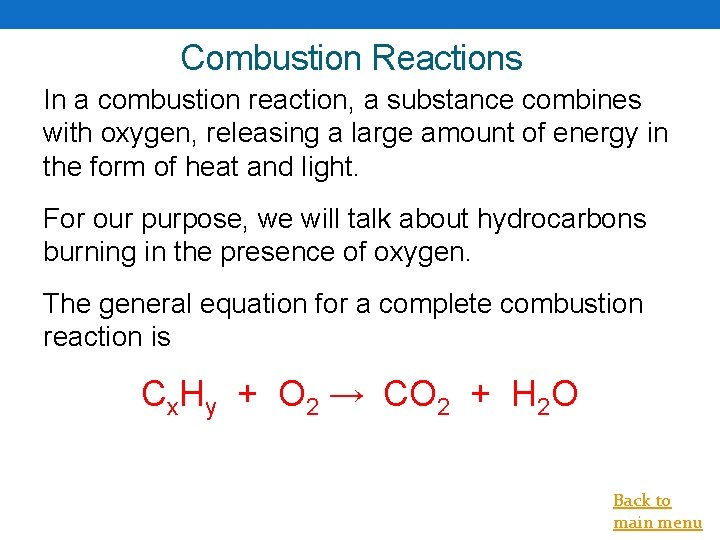
Combustion Reactions In a combustion reaction, a substance combines with oxygen, releasing a large amount of energy in the form of heat and light. For our purpose, we will talk about hydrocarbons burning in the presence of oxygen. The general equation for a complete combustion reaction is Cx. Hy + O 2 → CO 2 + H 2 O Back to main menu
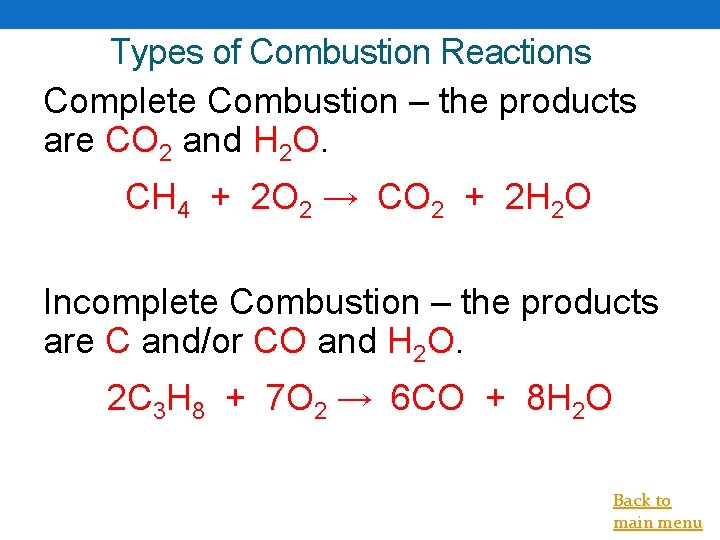
Types of Combustion Reactions Complete Combustion – the products are CO 2 and H 2 O. CH 4 + 2 O 2 → CO 2 + 2 H 2 O Incomplete Combustion – the products are C and/or CO and H 2 O. 2 C 3 H 8 + 7 O 2 → 6 CO + 8 H 2 O Back to main menu
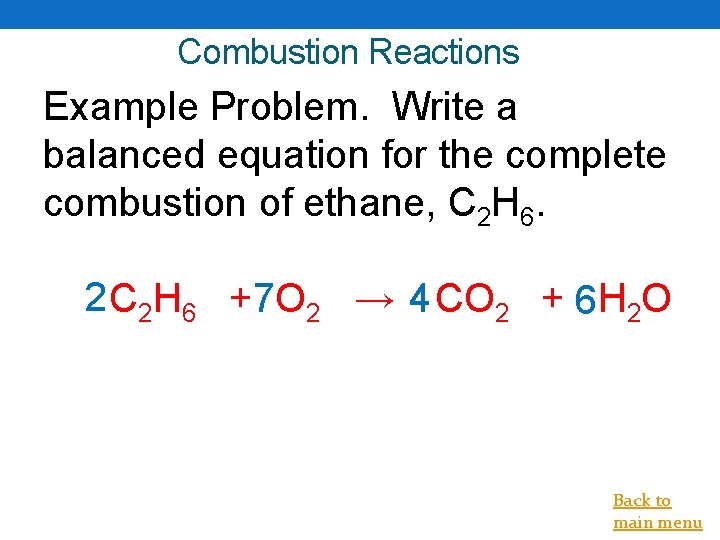
Combustion Reactions Example Problem. Write a balanced equation for the complete combustion of ethane, C 2 H 6. 2 C 2 H 6 +7 O 2 → 4 CO 2 + 6 H 2 O Back to main menu
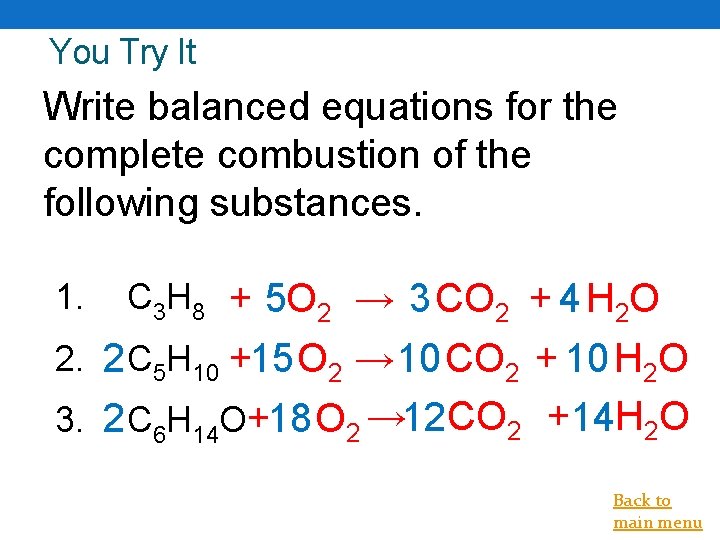
You Try It Write balanced equations for the complete combustion of the following substances. 1. C 3 H 8 + 5 O 2 → 3 CO 2 + 4 H 2 O 2. 2 C 5 H 10 +15 O 2 → 10 CO 2 + 10 H 2 O 3. 2 C 6 H 14 O+18 O 2 → 12 CO 2 +14 H 2 O Back to main menu
Single-Replacement Reactions In a single-replacement reaction, an uncombined element replaces an element in a compound. There are three general equations for a single replacement reaction. A + BC → AC + B (If A is a metal) Example: Zn + 2 HCl → Zn. Cl 2 + H 2 Back to main menu
Single-Replacement Reactions In a single-replacement reaction, an uncombined element replaces an element in a compound. There are three general equations for a single replacement reaction. A + BC → BA + C (If A is a halogen) Example: Cl 2 + 2 Li. Br → 2 Li. Cl + Br 2 Back to main menu
Single-Replacement Reactions In a single-replacement reaction, an uncombined element replaces an element in a compound. There are three general equations for a single replacement reaction. A + BC → Base + H 2 (If A is an active metal and BC is H 2 O) 2 K + 2 H 2 O → 2 KOH + H 2 Back to main menu
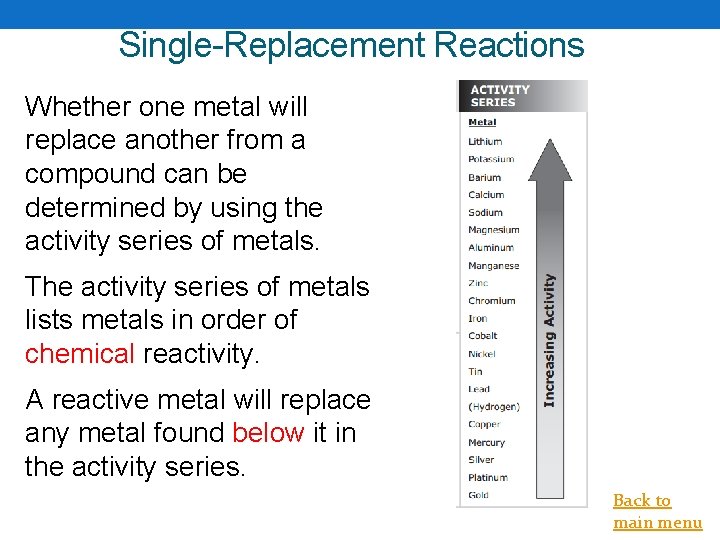
Single-Replacement Reactions Whether one metal will replace another from a compound can be determined by using the activity series of metals. The activity series of metals lists metals in order of chemical reactivity. A reactive metal will replace any metal found below it in the activity series. Back to main menu

Single-Replacement Reactions The halogens can also take place in single-replacement reactions. The order of reactivity for the halogens from increasing to decreasing reactivity is fluorine, chlorine, bromine, iodine. Back to main menu
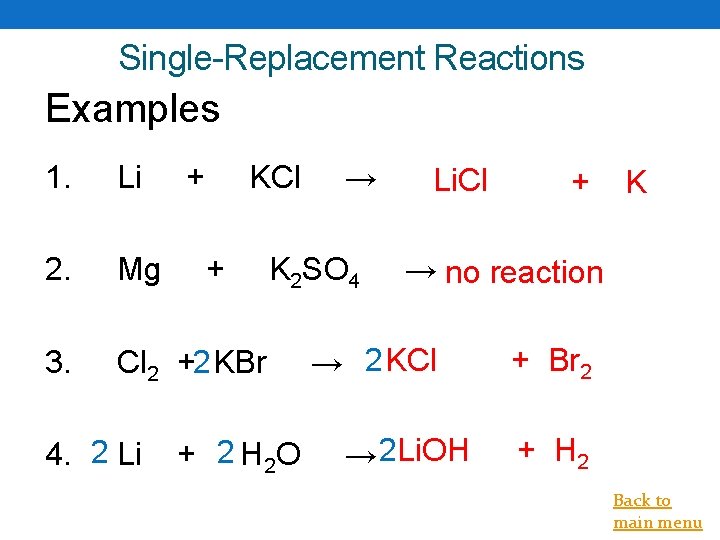
Single-Replacement Reactions Examples 1. Li 2. Mg 3. Cl 2 +2 KBr 4. 2 Li + KCl + → K 2 SO 4 + 2 H 2 O Li. Cl + K → no reaction → 2 KCl → 2 Li. OH + Br 2 + H 2 Back to main menu
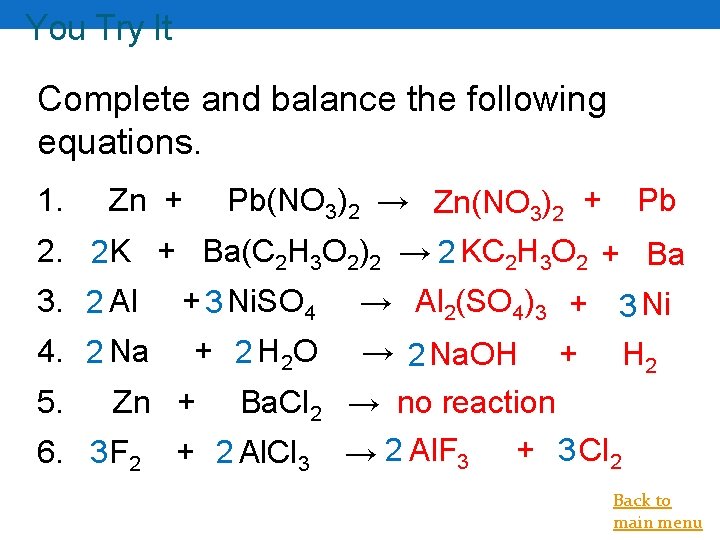
You Try It Complete and balance the following equations. 1. Pb(NO 3)2 → Zn(NO 3)2 + Zn + Pb 2. 2 K + Ba(C 2 H 3 O 2)2 → 2 KC 2 H 3 O 2 + Ba 3. 2 Al + 3 Ni. SO 4 → Al 2(SO 4)3 + 3 Ni 4. 2 Na 5. + 2 H 2 O Zn + 6. 3 F 2 Ba. Cl 2 + 2 Al. Cl 3 → 2 Na. OH + H 2 → no reaction → 2 Al. F 3 + 3 Cl 2 Back to main menu
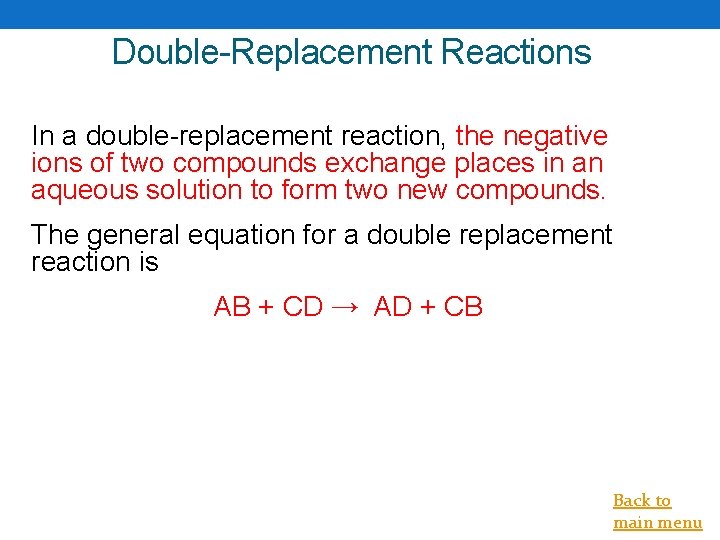
Double-Replacement Reactions In a double-replacement reaction, the negative ions of two compounds exchange places in an aqueous solution to form two new compounds. The general equation for a double replacement reaction is AB + CD → AD + CB Back to main menu
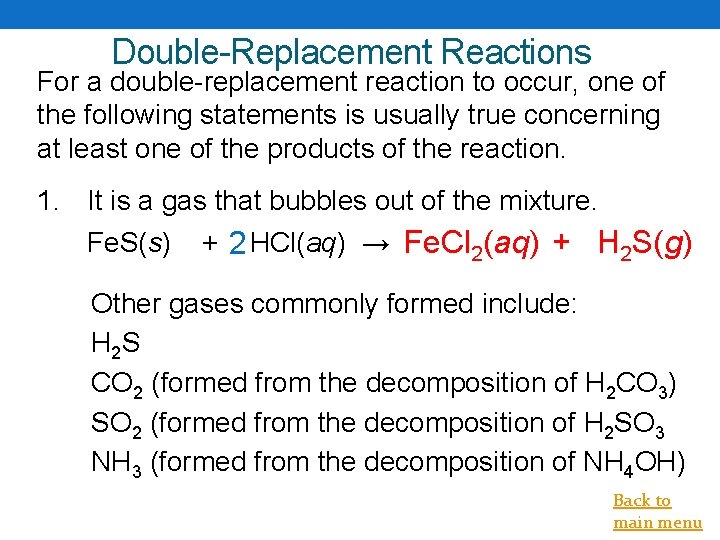
Double-Replacement Reactions For a double-replacement reaction to occur, one of the following statements is usually true concerning at least one of the products of the reaction. 1. It is a gas that bubbles out of the mixture. Fe. S(s) + 2 HCl(aq) → Fe. Cl 2(aq) + H 2 S(g) Other gases commonly formed include: H 2 S CO 2 (formed from the decomposition of H 2 CO 3) SO 2 (formed from the decomposition of H 2 SO 3 NH 3 (formed from the decomposition of NH 4 OH) Back to main menu
Double-Replacement Reactions 2. It is a molecular compound such as water. This is common in acid-base neutralization reactions. HCl(aq) + Na. OH(aq) → H 2 O(l) + Na. Cl(aq) Back to main menu
Double-Replacement Reactions 3. It is only slightly soluble and precipitates from solution. 2 KI(aq) + Pb(NO 3)2(aq) → 2 KNO 3(aq) + Pb. I 2(s) Back to main menu
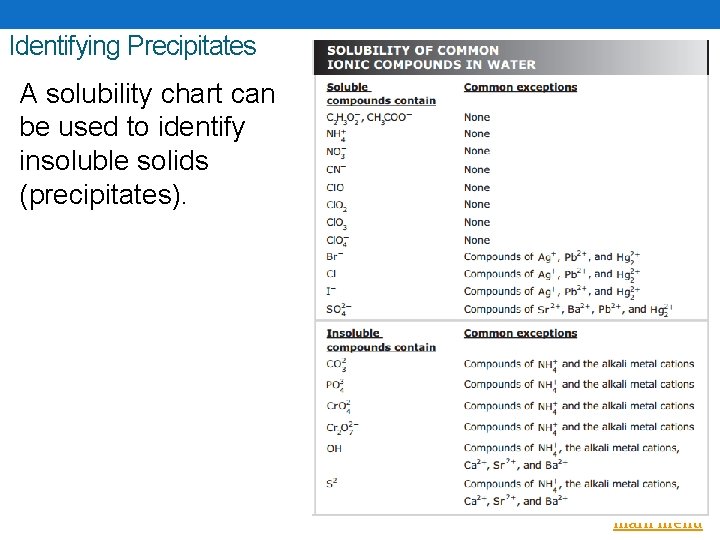
Identifying Precipitates A solubility chart can be used to identify insoluble solids (precipitates). Back to main menu
Identifying Precipitates Back to main menu Example. Label the precipitate formed in the following reaction. Na 2 CO 3(aq) + Ca(NO 3)2(aq) → 2 Na. NO 3( aq) + Ca. CO 3( s )
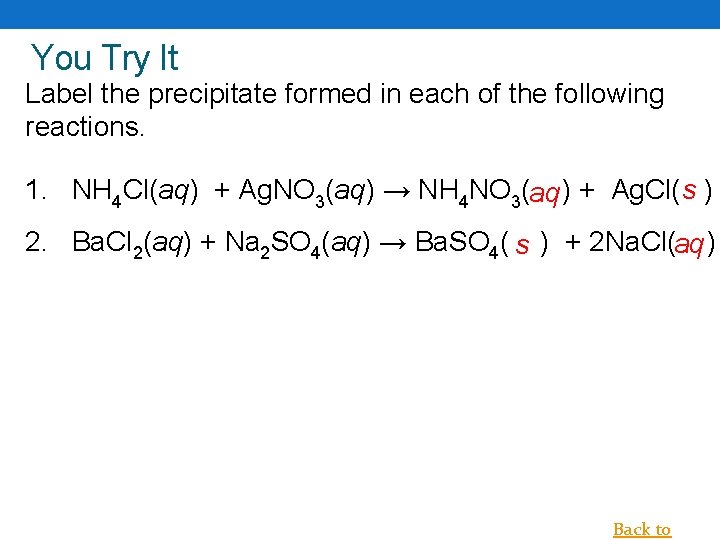
You Try It Label the precipitate formed in each of the following reactions. 1. NH 4 Cl(aq) + Ag. NO 3(aq) → NH 4 NO 3(aq ) + Ag. Cl( s ) 2. Ba. Cl 2(aq) + Na 2 SO 4(aq) → Ba. SO 4( s ) + 2 Na. Cl(aq ) Back to
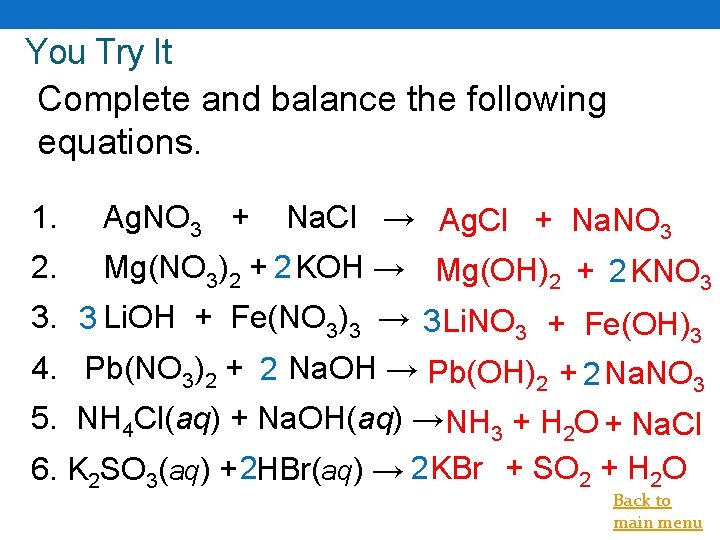
You Try It Complete and balance the following equations. 1. Ag. NO 3 + Na. Cl → Ag. Cl + Na. NO 3 2. Mg(NO 3)2 + 2 KOH → Mg(OH)2 + 2 KNO 3 3. 3 Li. OH + Fe(NO 3)3 → 3 Li. NO 3 + Fe(OH)3 4. Pb(NO 3)2 + 2 Na. OH → Pb(OH)2 + 2 Na. NO 3 5. NH 4 Cl(aq) + Na. OH(aq) → NH 3 + H 2 O + Na. Cl 6. K 2 SO 3(aq) + 2 HBr(aq) → 2 KBr + SO 2 + H 2 O Back to main menu

Oxidation-Reduction Reactions (Redox) Oxidation-Reduction reactions are the chemical changes that occur when electrons are transferred between reactions. Examples include the burning of gasoline and the rusting of a nail. Back to main menu
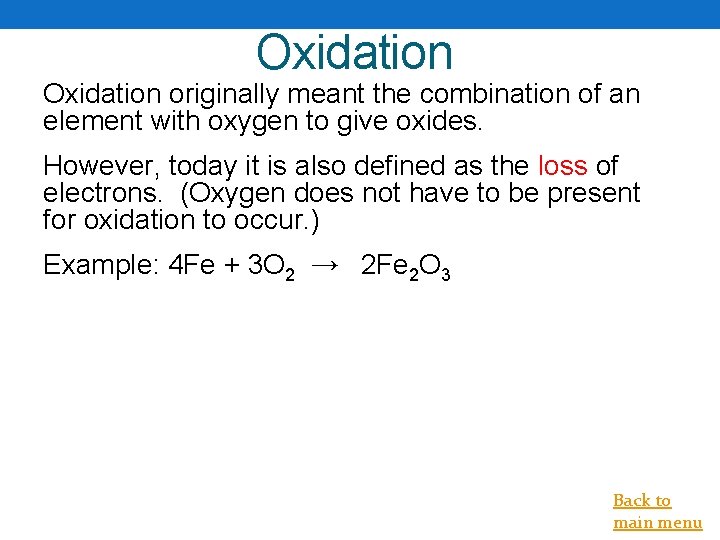
Oxidation originally meant the combination of an element with oxygen to give oxides. However, today it is also defined as the loss of electrons. (Oxygen does not have to be present for oxidation to occur. ) Example: 4 Fe + 3 O 2 → 2 Fe 2 O 3 Back to main menu
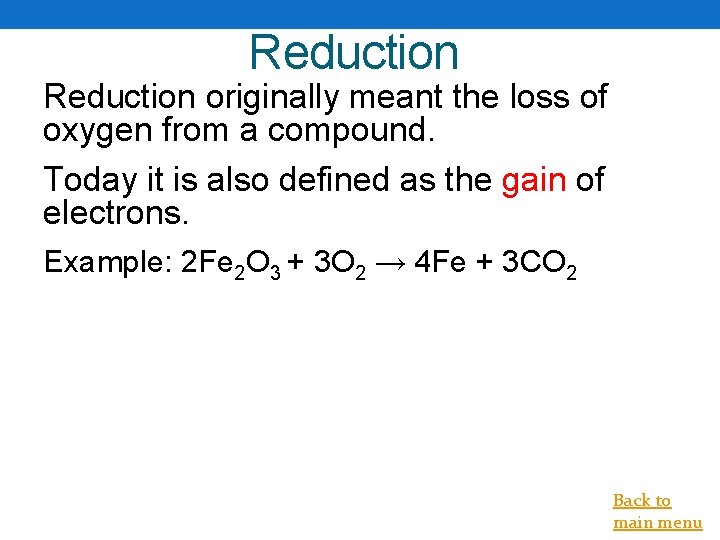
Reduction originally meant the loss of oxygen from a compound. Today it is also defined as the gain of electrons. Example: 2 Fe 2 O 3 + 3 O 2 → 4 Fe + 3 CO 2 Back to main menu

Oxidizing Agent The oxidizing agent in a redox reaction gains electrons. It is the substance being reduced. Back to main menu

Reducing Agent The reducing agent loses electrons. It is the substance being oxidized. Back to main menu
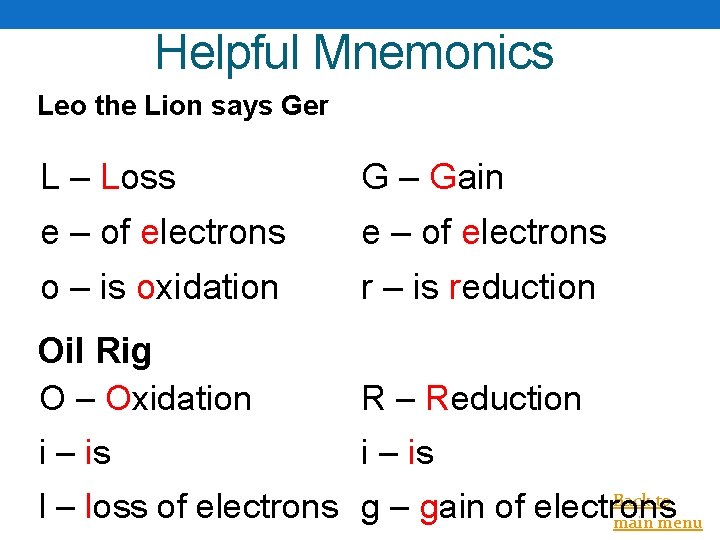
Helpful Mnemonics Leo the Lion says Ger L – Loss G – Gain e – of electrons o – is oxidation r – is reduction Oil Rig O – Oxidation R – Reduction i – is Back to l – loss of electrons g – gain of electrons main menu
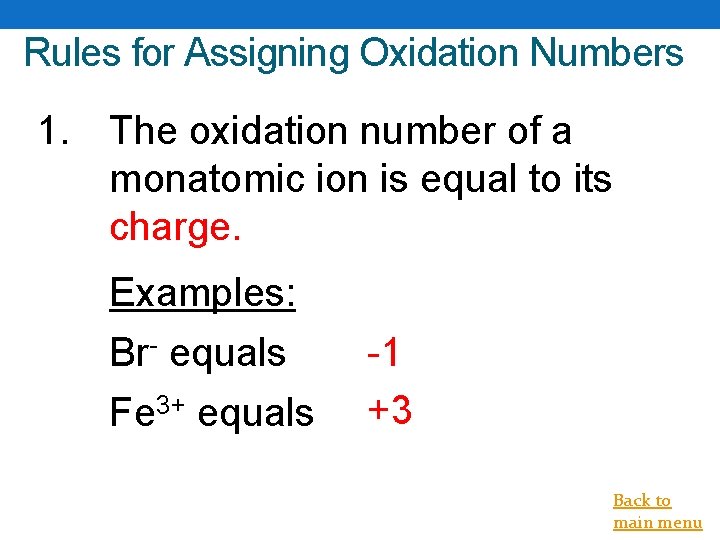
Rules for Assigning Oxidation Numbers 1. The oxidation number of a monatomic ion is equal to its charge. Examples: Br- equals Fe 3+ equals -1 +3 Back to main menu
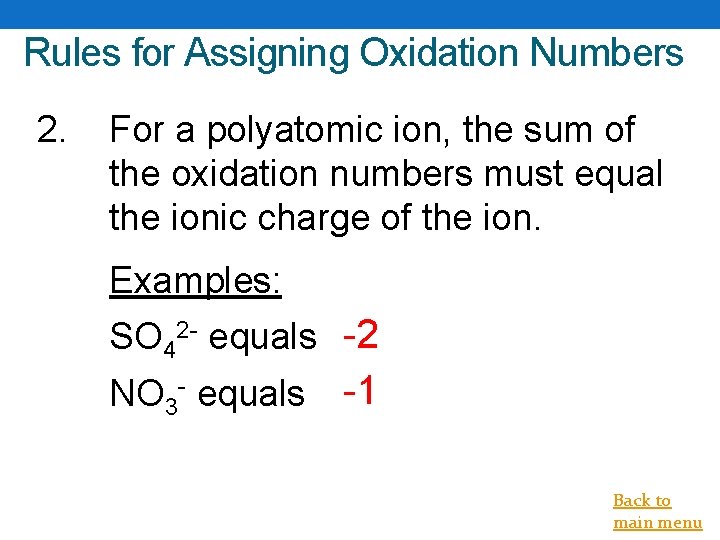
Rules for Assigning Oxidation Numbers 2. For a polyatomic ion, the sum of the oxidation numbers must equal the ionic charge of the ion. Examples: SO 42 - equals -2 NO 3 - equals -1 Back to main menu

Rules for Assigning Oxidation Numbers 3. The oxidation number of a metal cation is the same as its ionic charge. Examples: sodium is calcium is +1 +2 Back to main menu

Rules for Assigning Oxidation Numbers 4. The oxidation number of hydrogen in a compound is +1 except in metal hydrides, for example, Na. H, where it is -1. Back to main menu

Rules for Assigning Oxidation Numbers 5. The oxidation number of oxygen in a compound is -2 except in peroxides, for example, H 2 O 2, where it is -1. Back to main menu
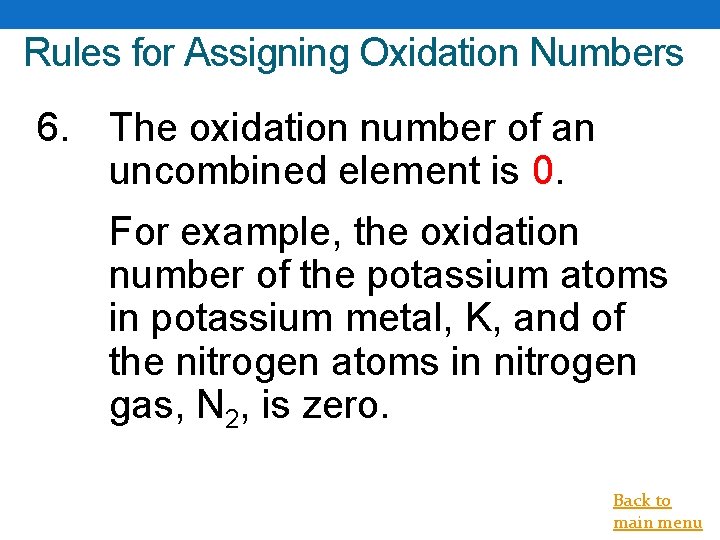
Rules for Assigning Oxidation Numbers 6. The oxidation number of an uncombined element is 0. For example, the oxidation number of the potassium atoms in potassium metal, K, and of the nitrogen atoms in nitrogen gas, N 2, is zero. Back to main menu

Rules for Assigning Oxidation Numbers 7. For any neutral compound, the sum of the oxidation numbers of the atoms in the compound must equal 0. Back to main menu
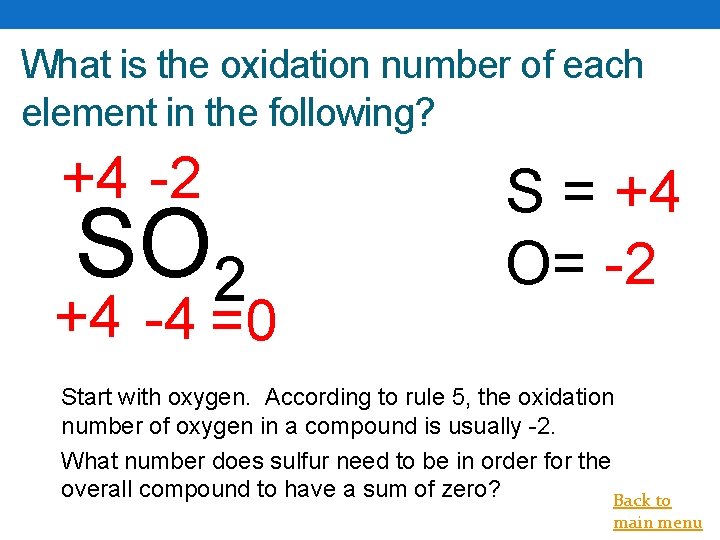
What is the oxidation number of each element in the following? +4 -2 SO 2 +4 -4 =0 S = +4 O= -2 Start with oxygen. According to rule 5, the oxidation number of oxygen in a compound is usually -2. What number does sulfur need to be in order for the overall compound to have a sum of zero? Back to main menu
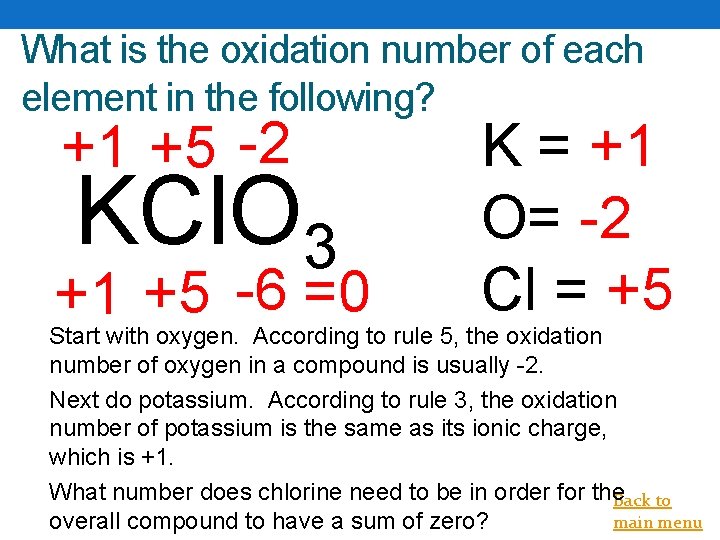
What is the oxidation number of each element in the following? +1 +5 -2 KCl. O 3 +1 +5 -6 =0 K = +1 O= -2 Cl = +5 Start with oxygen. According to rule 5, the oxidation number of oxygen in a compound is usually -2. Next do potassium. According to rule 3, the oxidation number of potassium is the same as its ionic charge, which is +1. What number does chlorine need to be in order for the. Back to main menu overall compound to have a sum of zero?
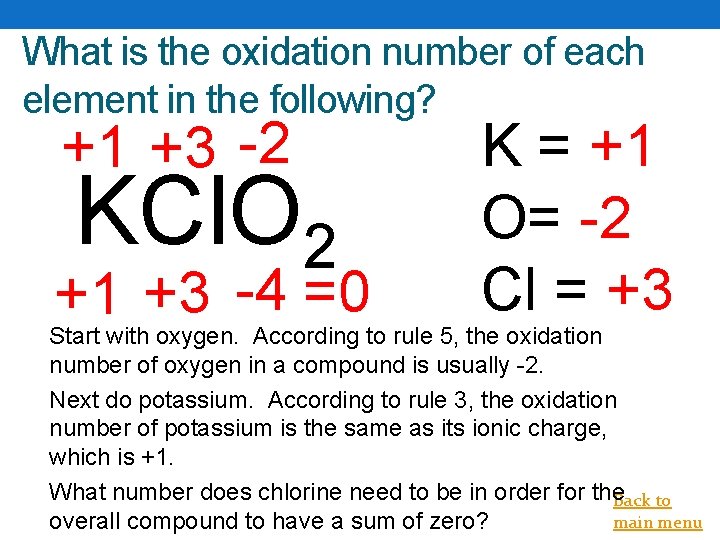
What is the oxidation number of each element in the following? +1 +3 -2 KCl. O 2 +1 +3 -4 =0 K = +1 O= -2 Cl = +3 Start with oxygen. According to rule 5, the oxidation number of oxygen in a compound is usually -2. Next do potassium. According to rule 3, the oxidation number of potassium is the same as its ionic charge, which is +1. What number does chlorine need to be in order for the. Back to main menu overall compound to have a sum of zero?
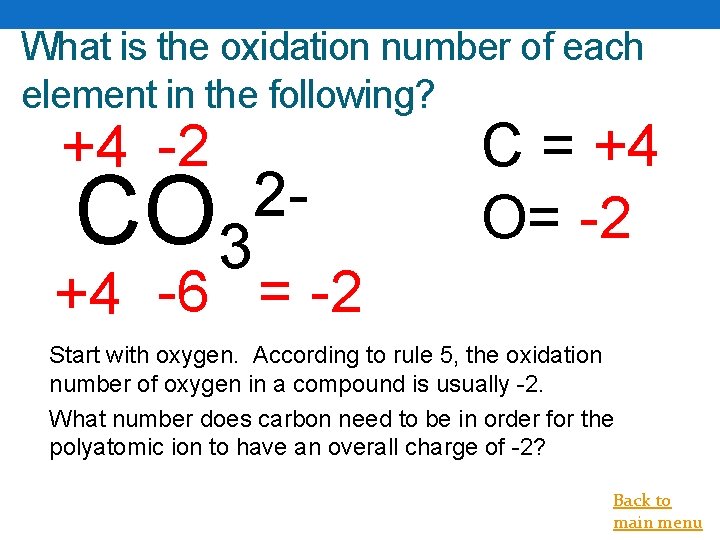
What is the oxidation number of each element in the following? +4 -2 CO 3 2 - C = +4 O= -2 +4 -6 = -2 Start with oxygen. According to rule 5, the oxidation number of oxygen in a compound is usually -2. What number does carbon need to be in order for the polyatomic ion to have an overall charge of -2? Back to main menu
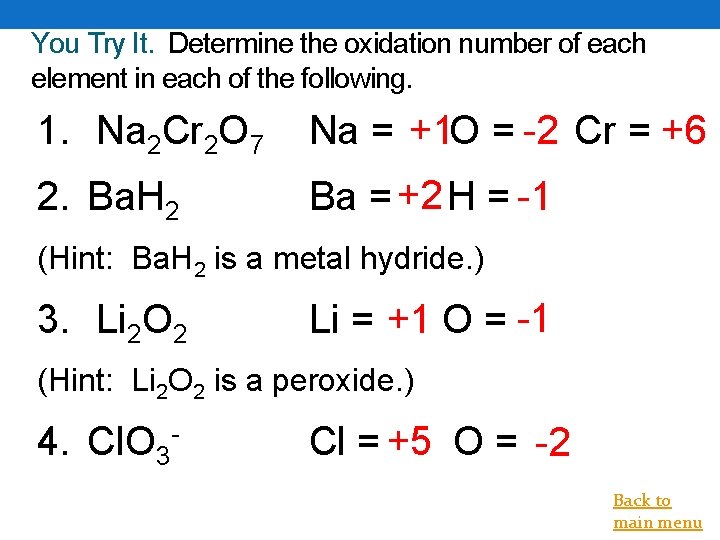
You Try It. Determine the oxidation number of each element in each of the following. 1. Na 2 Cr 2 O 7 Na = +1 O = -2 Cr = +6 2. Ba. H 2 Ba = +2 H = -1 (Hint: Ba. H 2 is a metal hydride. ) 3. Li 2 O 2 Li = +1 O = -1 (Hint: Li 2 O 2 is a peroxide. ) 4. Cl. O 3 - Cl = +5 O = -2 Back to main menu
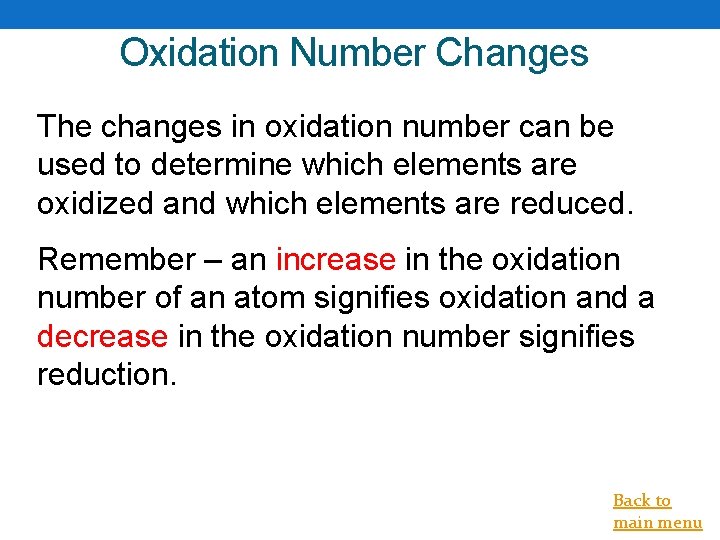
Oxidation Number Changes The changes in oxidation number can be used to determine which elements are oxidized and which elements are reduced. Remember – an increase in the oxidation number of an atom signifies oxidation and a decrease in the oxidation number signifies reduction. Back to main menu
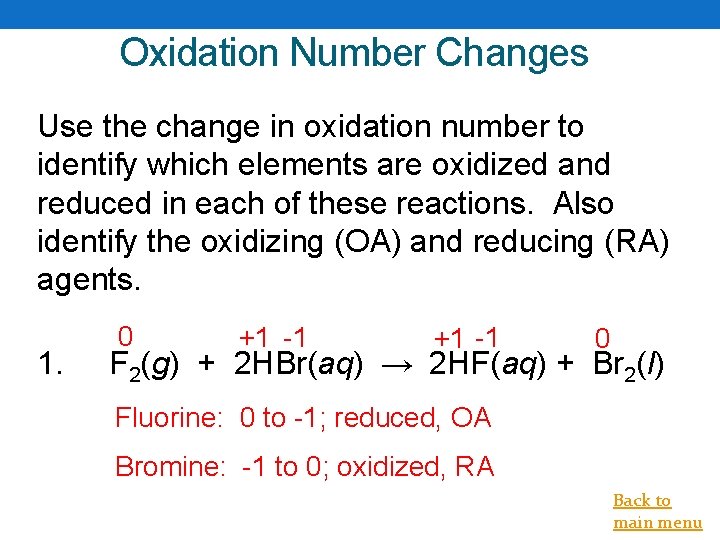
Oxidation Number Changes Use the change in oxidation number to identify which elements are oxidized and reduced in each of these reactions. Also identify the oxidizing (OA) and reducing (RA) agents. 1. 0 +1 -1 0 F 2(g) + 2 HBr(aq) → 2 HF(aq) + Br 2(l) Fluorine: 0 to -1; reduced, OA Bromine: -1 to 0; oxidized, RA Back to main menu
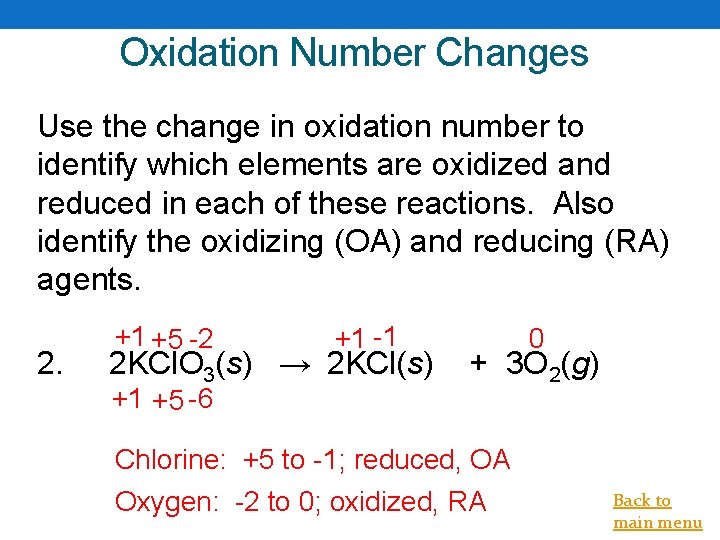
Oxidation Number Changes Use the change in oxidation number to identify which elements are oxidized and reduced in each of these reactions. Also identify the oxidizing (OA) and reducing (RA) agents. 2. +1 +5 -2 +1 -1 2 KCl. O 3(s) → 2 KCl(s) +1 +5 -6 0 + 3 O 2(g) Chlorine: +5 to -1; reduced, OA Oxygen: -2 to 0; oxidized, RA Back to main menu
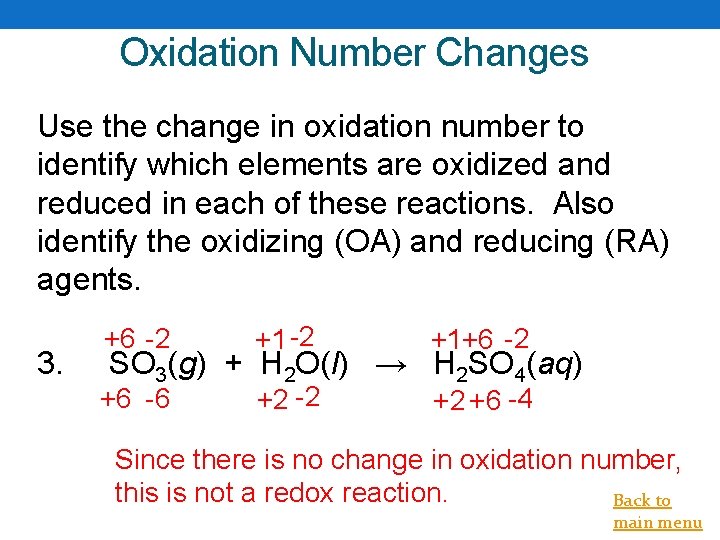
Oxidation Number Changes Use the change in oxidation number to identify which elements are oxidized and reduced in each of these reactions. Also identify the oxidizing (OA) and reducing (RA) agents. 3. +6 -2 +1+6 -2 +6 -6 +2 -2 +2 +6 -4 SO 3(g) + H 2 O(l) → H 2 SO 4(aq) Since there is no change in oxidation number, this is not a redox reaction. Back to main menu
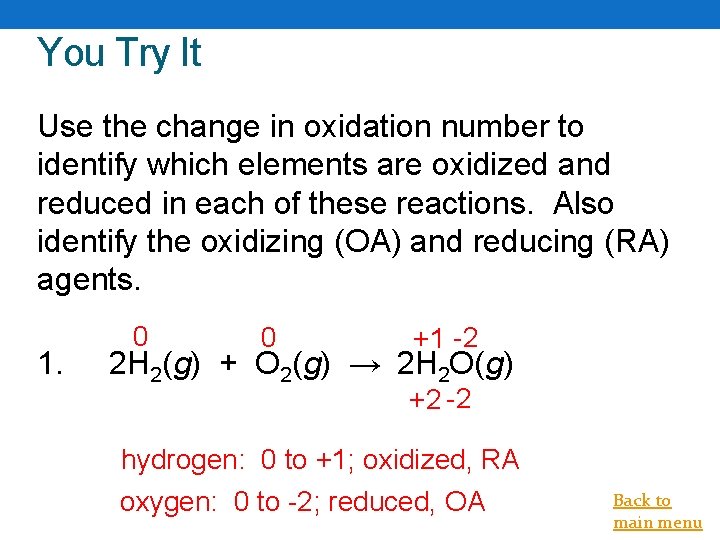
You Try It Use the change in oxidation number to identify which elements are oxidized and reduced in each of these reactions. Also identify the oxidizing (OA) and reducing (RA) agents. 1. 0 0 +1 -2 2 H 2(g) + O 2(g) → 2 H 2 O(g) +2 -2 hydrogen: 0 to +1; oxidized, RA oxygen: 0 to -2; reduced, OA Back to main menu
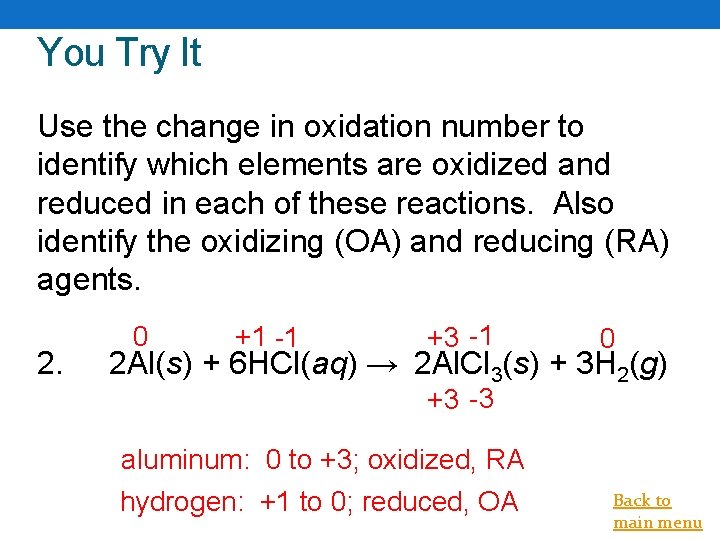
You Try It Use the change in oxidation number to identify which elements are oxidized and reduced in each of these reactions. Also identify the oxidizing (OA) and reducing (RA) agents. 2. 0 +1 -1 +3 -1 0 2 Al(s) + 6 HCl(aq) → 2 Al. Cl 3(s) + 3 H 2(g) +3 -3 aluminum: 0 to +3; oxidized, RA hydrogen: +1 to 0; reduced, OA Back to main menu
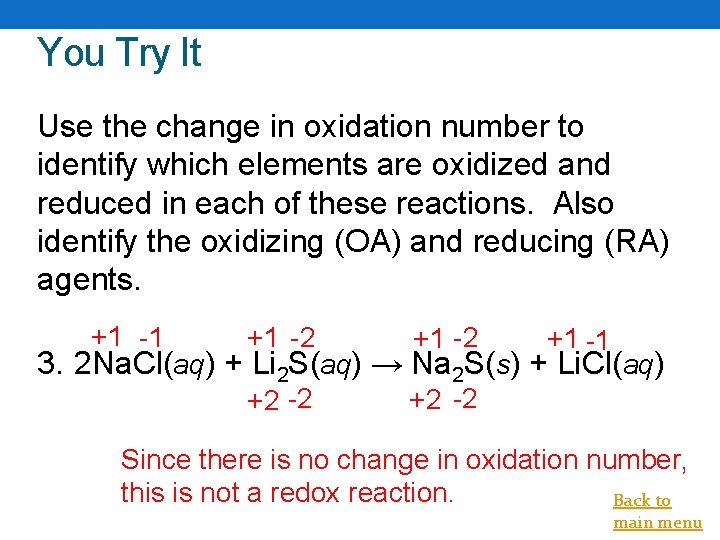
You Try It Use the change in oxidation number to identify which elements are oxidized and reduced in each of these reactions. Also identify the oxidizing (OA) and reducing (RA) agents. +1 -1 +1 -2 +2 -2 +1 -1 3. 2 Na. Cl(aq) + Li 2 S(aq) → Na 2 S(s) + Li. Cl(aq) Since there is no change in oxidation number, this is not a redox reaction. Back to main menu
- Slides: 85