Chemical Reactions Balancing Chemical Equations Changes Change usually
Chemical Reactions Balancing Chemical Equations

Changes • Change usually comes in two forms: PHYSICAL CHEMICAL Physical change occurs when only the form or shape of the matter changes but the composition stays the same (it looks different but contains the same atoms & molecules). Chemical change occurs when the composition of a substance changes into a different substance (the molecules & atoms change into different molecules).

What types of change happened here? B CHEMCIAL A PHYSICAL
6 Indicators of a Chemical Change • In your study of matter, you learned that there are three ways to determine if there was a chemical change; a change in the composition of a substance. • Recall, they were… – Change in color – Production of a gas – Formation of a precipitate • In total, there are 6 indicators of a chemical change; three more to truly be inclusive of a chemical change. – Production of light – Change in temperature (heat or cold) – Obvious change in mass

Energy & Change • The necessary ingredient of change is energy. • Energy is the ability to move or change matter. – You studied two major kinds of energy • Kinetic = the energy of motion • Potential = stored energy – Luckily, energy is all around us and exists in many forms. – It can be converted from one form to another & with enough education you can cause this conversion yourself, on purpose. – This is what Chemists do!

Forms of Energy • Kinetic (Thermal Heat): • Energy of motion • Potential: • Energy that can be used at a later time. • Mechanical: • Energy transferred in machines • Electro-magnetic: • Electrons transferring energy • Nuclear: • Energy stored in the nucleus of atoms • Sound: • Energy in waves • Chemical: • Energy stored in bonds

Changing Matter: Bound by Laws • There are many other constraints on changes in the natural world = Universal Laws • Law of Conservation of Mass. = Matter is neither created nor destroyed in any change. – This means that any new forms of matter comes from existing matter! • Entropy = As matter changes, some energy is lost as heat to the surroundings • This means with every change some energy is lost forever, requiring refueling. • Law of conservation of energy = Energy may change from one form to another, but the total amount of energy does not change. – This means that any new type of energy comes from energy that has already been here…just in a different form.
Chemical Reactions

Chemical Reactions • Changing a substance, chemically, requires a chemical reaction. • During a chemical reaction, bonds between atoms are broken and new ones are formed. • There are two participants of a reaction • A reactant (aka substrate) is a substance that is changed in a chemical reaction – It’s all the stuff that you start with that mixes and mingles together. • A product is a new substance that is formed. – This is the stuff that you end up with after the mingling.

Reactants to Products Reactants Reaction Products
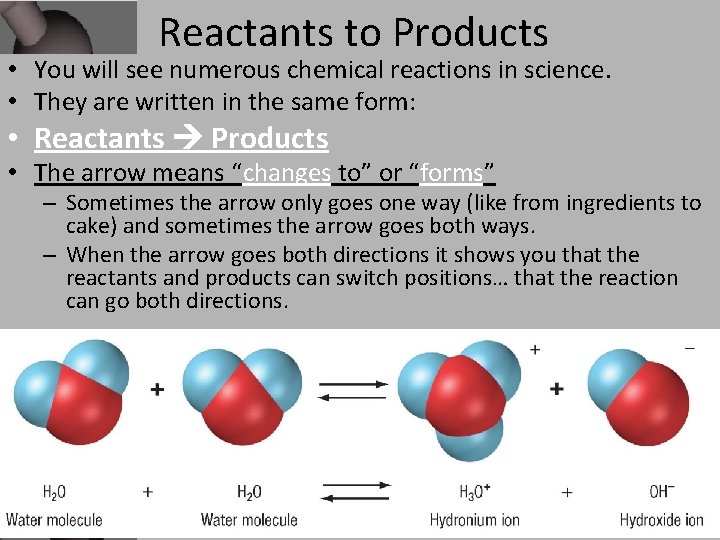
Reactants to Products • You will see numerous chemical reactions in science. • They are written in the same form: • Reactants Products • The arrow means “changes to” or “forms” – Sometimes the arrow only goes one way (like from ingredients to cake) and sometimes the arrow goes both ways. – When the arrow goes both directions it shows you that the reactants and products can switch positions… that the reaction can go both directions.
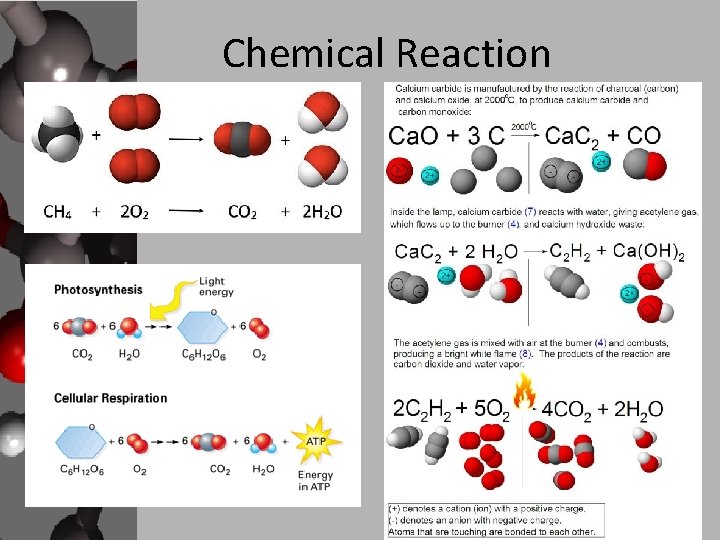
Chemical Reaction
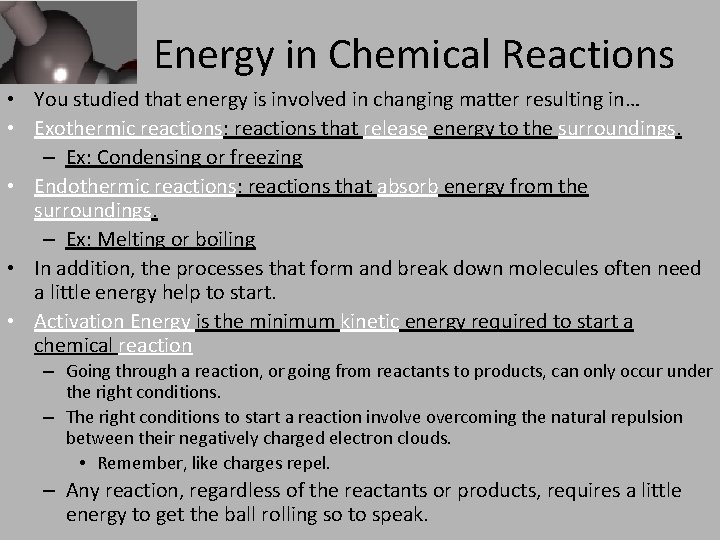
Energy in Chemical Reactions • You studied that energy is involved in changing matter resulting in… • Exothermic reactions: reactions that release energy to the surroundings. – Ex: Condensing or freezing • Endothermic reactions: reactions that absorb energy from the surroundings. – Ex: Melting or boiling • In addition, the processes that form and break down molecules often need a little energy help to start. • Activation Energy is the minimum kinetic energy required to start a chemical reaction – Going through a reaction, or going from reactants to products, can only occur under the right conditions. – The right conditions to start a reaction involve overcoming the natural repulsion between their negatively charged electron clouds. • Remember, like charges repel. – Any reaction, regardless of the reactants or products, requires a little energy to get the ball rolling so to speak.
Types of Chemical Reactions • Taken from the Law of the Conservation of Matter, atoms in a chemical reaction are neither created nor destroyed. • They are simply rearranged in several ways to give us the three major categories of chemical reactions. • Synthesis: when two or more atoms are bonded together to make something bigger. • Decomposition: when a larger substance is chemically broken down into different smaller parts. • Replacement: When atoms from one reactant are taken away and re-bonded with other reactants in the reaction to make a new product.
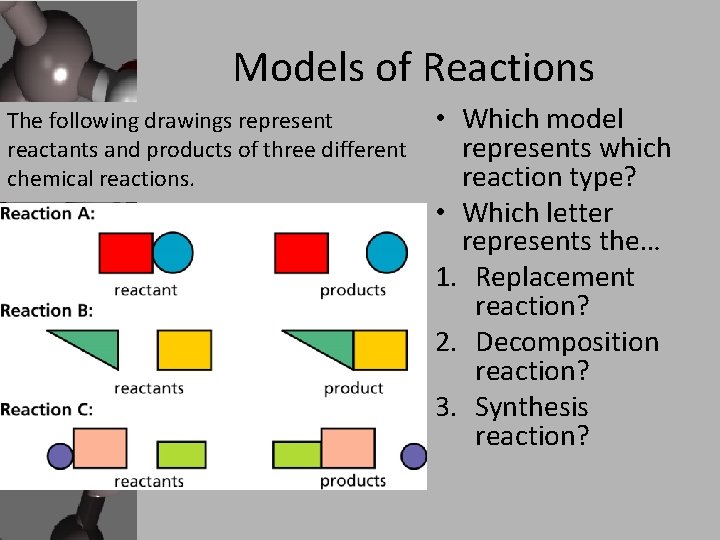
Models of Reactions The following drawings represent reactants and products of three different chemical reactions. • Which model represents which reaction type? • Which letter represents the… 1. Replacement reaction? 2. Decomposition reaction? 3. Synthesis reaction?
Chemical Reactions and their Equations. • In a chemical reaction, products combine, rearrange, or break down into different products, but the starting number of atoms in the reactants must equal the number of atoms in the products. • Chemical Equations help us ensure the proper amounts of atoms are accounted for in a reaction. • Therefore, a chemical equation is a written, balanced representation of a chemical reaction.
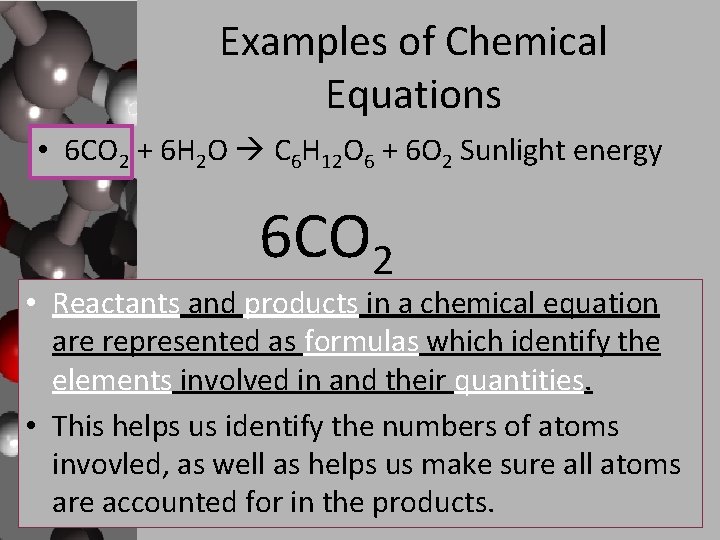
Examples of Chemical Equations • 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2 Sunlight energy 6 CO 2 • Reactants and products in a chemical equation are represented as formulas which identify the elements involved in and their quantities. • This helps us identify the numbers of atoms invovled, as well as helps us make sure all atoms are accounted for in the products.
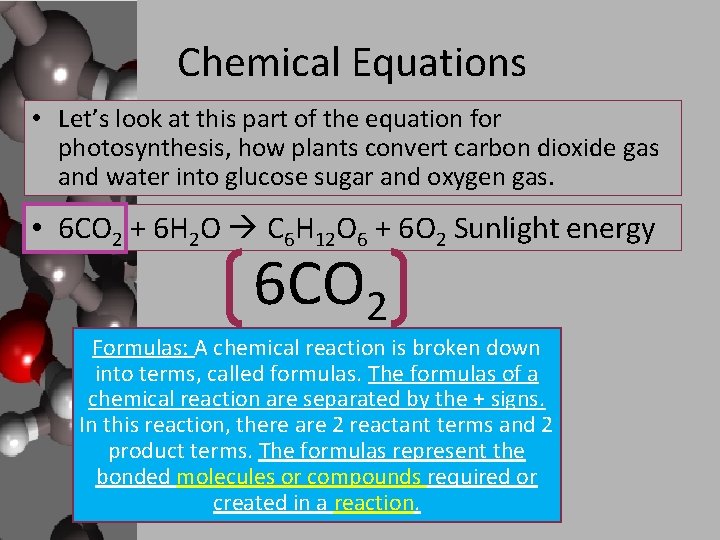
Chemical Equations • Let’s look at this part of the equation for photosynthesis, how plants convert carbon dioxide gas and water into glucose sugar and oxygen gas. • 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2 Sunlight energy 6 CO 2 Formulas: A chemical reaction is broken down into terms, called formulas. The formulas of a chemical reaction are separated by the + signs. In this reaction, there are 2 reactant terms and 2 product terms. The formulas represent the bonded molecules or compounds required or created in a reaction.

Breaking Down Formulas in Chemical Equations • Let’s look at this part of the equation for photosynthesis, how plants convert carbon dioxide gas and water into glucose sugar and oxygen gas. • 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2 Sunlight energy 6 CO 2 Subscript: You Coefficient: You identify the quantity of the elements of the bonded involved in the molecules/ You identify the compounds by the elements involved molecule/compound by the little number in front of by looking at the formula. capital letters which hanging from the bottom. represent the element symbols.

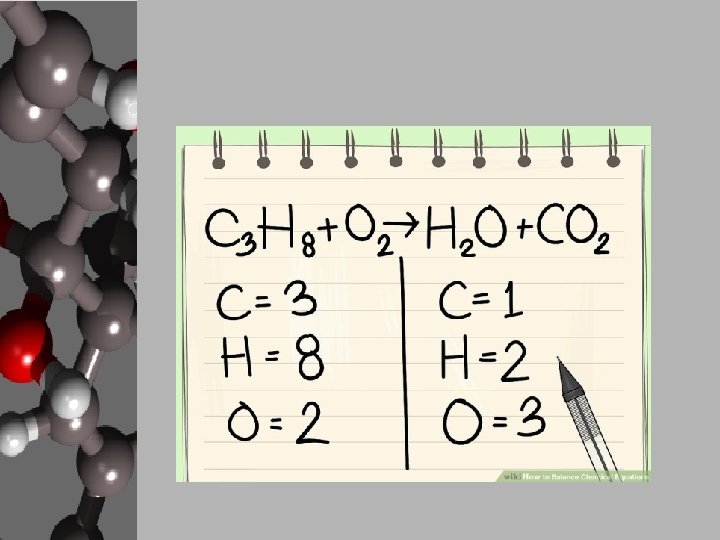
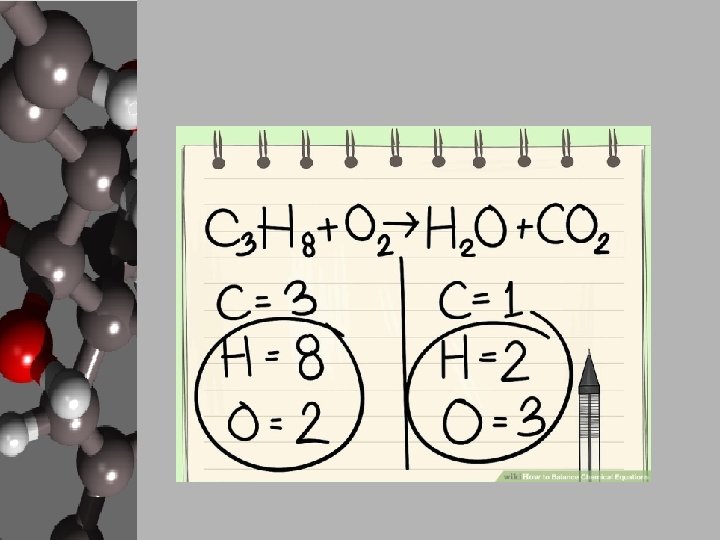
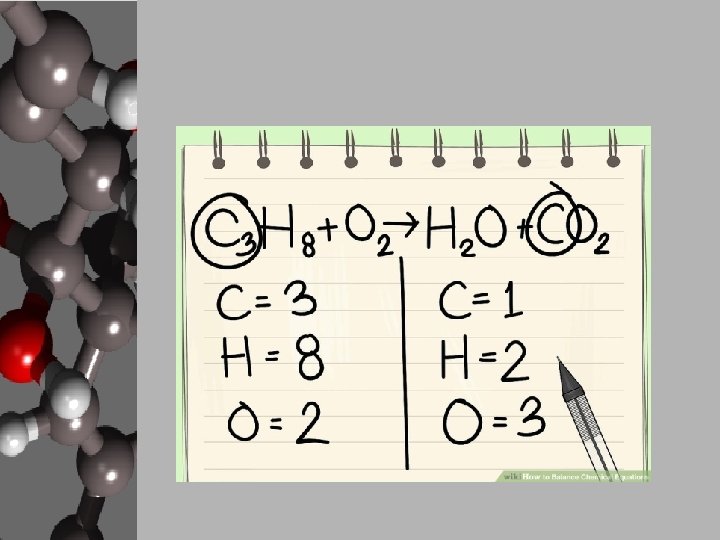
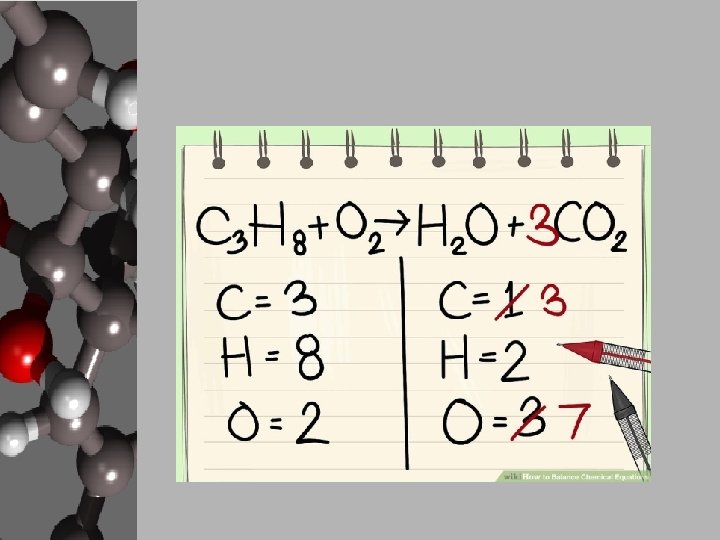
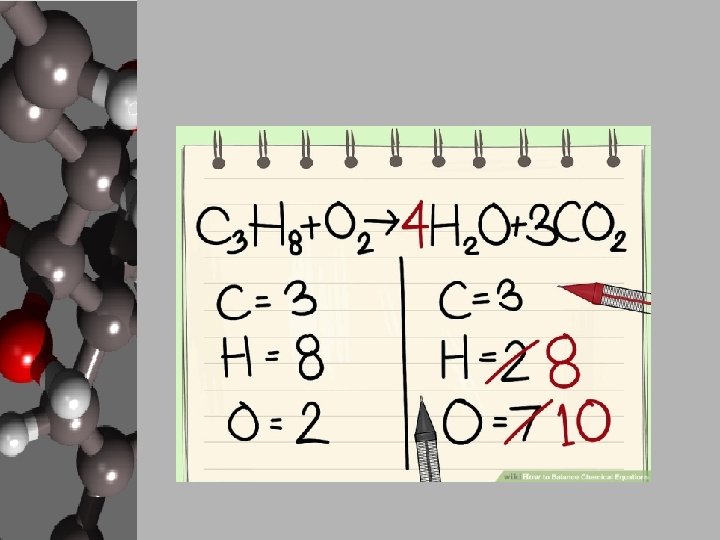
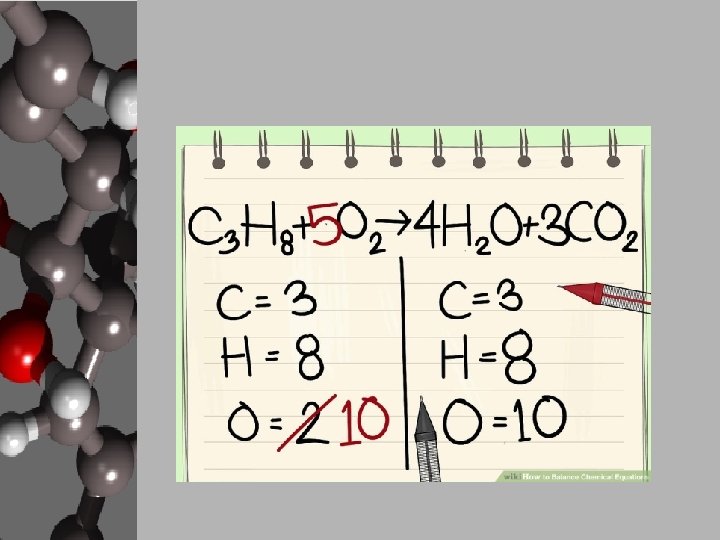
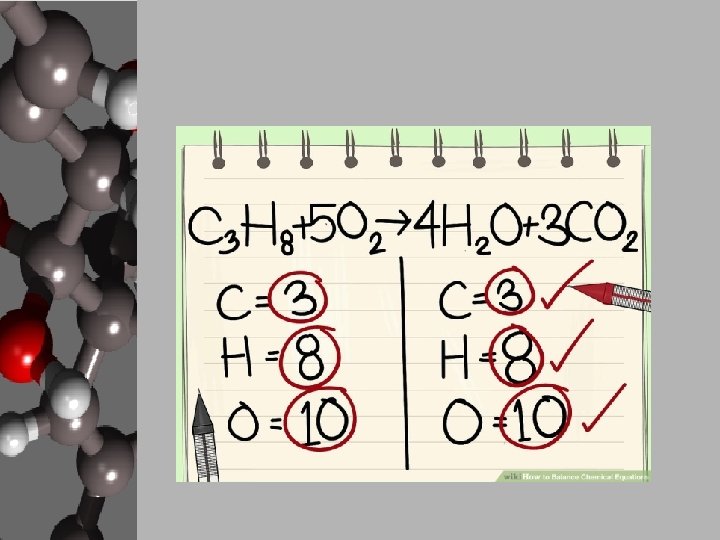
Your Goal: Balancing Basic Chemical Reactions • In order to create products, chemists use chemical equations like cooks use recipes. • Chemists decide what they want to create and how much of it then either know, search out, or create a chemical equation that will give the proper reaction. • They gather the materials, add the necessary energy, and conduct a process for the products to form. • These reactions can be found, but your goal is to apply the Law of Conservation of Matter and balance equations. • Luckily, there are steps to follow to make this happen.
Steps to Balancing Basic Equations • In order to balance equations while applying the LCM, follow these steps… 1. Start with what’s given to determine the number of atoms for each element. USE A REP TABLE. 2. Pick an element that is obviously not equal on both sides of the equation. 3. Add a coefficient in front of the formula with that element and adjust your counts. 4. Continue adding coefficients to get the same number of atoms of each element on each side.

Let’s Practice! • Using the “Balancing Act” worksheet, let’s balance the given equation. ____Mg + ____O 2 ____ Mg O Reactants 1. 2. 3. 4. Elements Products Start with what’s given to determine the number of atoms for each element. USE A REP TABLE. Pick an element that is obviously not equal on both sides of the equation. Add a coefficient in front of the formula with that element and adjust your counts. Continue adding coefficients to get the same number of atoms of each element on each side.

Finished Product • Easy, right? ! 2 Mg + (1) O 2 2 Mg O

Practice With the Next Examples • Complete the “Try these: ” problems. • You have 10 minutes. • Ask questions as needed!



- Slides: 34