Chemical Reactions Balance Equation Doubledisplacement Reaction q Precipitation
Chemical Reactions • Balance Equation • Double-displacement Reaction: q Precipitation q acid-base neutralization q gas-evolving • Single-displacement Reaction

Experiencing Chemical Change Chemical Reactions: happening both around you and in you all the time! + • some are very simple, others are complex • involving changes in the structures of the molecules many times we can experience the effects of those changes Chemical reactions you experience? • Combustion of gasoline/natural gas • Seasonal color change of leaves • Bleaching of laundry 2
Chemical Reactions • Chemical changes: New matter forms • Rearrangement and exchange of atoms to produce new molecules üElements are not transmuted A reaction resemble life cycles (at 1: 45) Reactants Products 3
Chemical Equations: Conservation of Mass • Matter cannot be created or destroyed üTotal mass cannot change üTotal mass of the reactants will be the same as the total mass of the products: Mass Reactant = Mass Product üAll the atoms present at the beginning are still present at the end: #Atom X (Reactant) = #Atom X (Product) üif all the atoms are still there, then the mass will 4 not change
Components in Chemical Equations: 2 Mg(s) + O 2(g) 2 Mg. O(s) • Shorthand way of describing a reaction • Provides information about the reaction üFormulas of Reactants (Left) and Products (Right) üStates of Reactants and Products in “( )”. (solid/liquid/gas) üRelative #Reactant and #Product molecules (Coefficients) 5

Symbols Used in Equations • Indicating the state after chemical ü(g) = gas; (l) = liquid; (s) = solid ü(aq) = aqueous = dissolved in water • energy symbols used above the arrow for decomposition reactions ü D = heat ü hn = light üshock = mechanical üelec = electrical 6 6
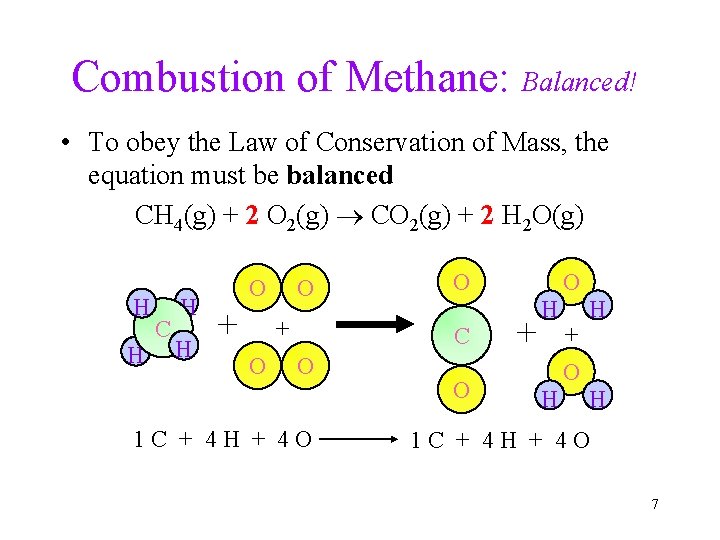
Combustion of Methane: Balanced! • To obey the Law of Conservation of Mass, the equation must be balanced CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) H H C H H O + O O C O 1 C + 4 H + 4 O O + H H O + O H H 1 C + 4 H + 4 O 7
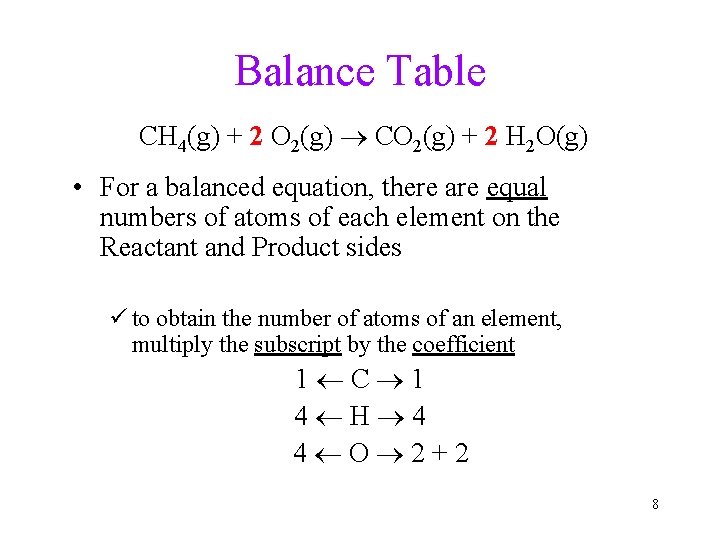
Balance Table CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) • For a balanced equation, there are equal numbers of atoms of each element on the Reactant and Product sides ü to obtain the number of atoms of an element, multiply the subscript by the coefficient 1 C 1 4 H 4 4 O 2+2 8

How to Write & Balance Chemical Equation __CH 4 O(g) + __O 2(g) __CO 2(g) + __H 2 O(g) 1. 2. 3. Skeletal equation Balance table: Polyatomic ions as one “Group”. Pick an element to balance: ü Pick Element/Group w/ highest #Atoms 4. Find the Least Common Multiple 5. Multiply each count by a factor to equal the LCM. 6. Recount and Repeat until Balanced. 9

Example Solid aluminum react with aqueous sulfuric acid to form aqueous aluminum sulfate and hydrogen gas 1. Write a skeletal equation. 2. Count #Atom or #Groups 3. Setup balancing table and balance. Don’t start with elements in more than one formula 4. Find the Least Common Multiple of the number of atoms on each side. 10
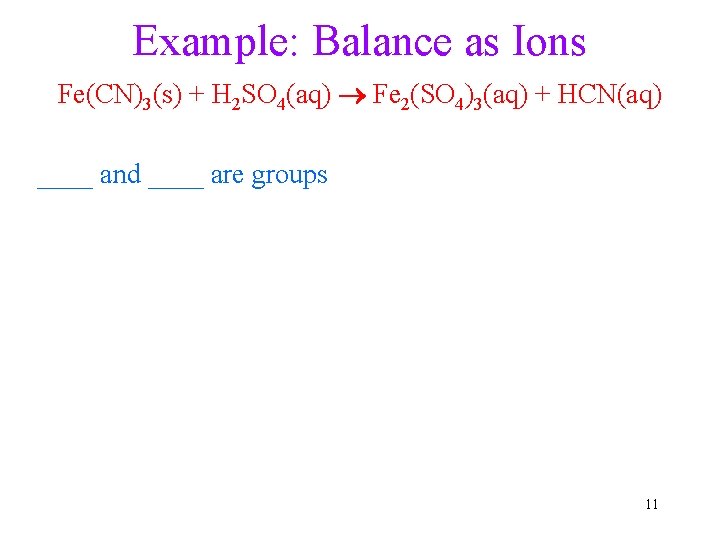
Example: Balance as Ions Fe(CN)3(s) + H 2 SO 4(aq) Fe 2(SO 4)3(aq) + HCN(aq) ____ and ____ are groups 11
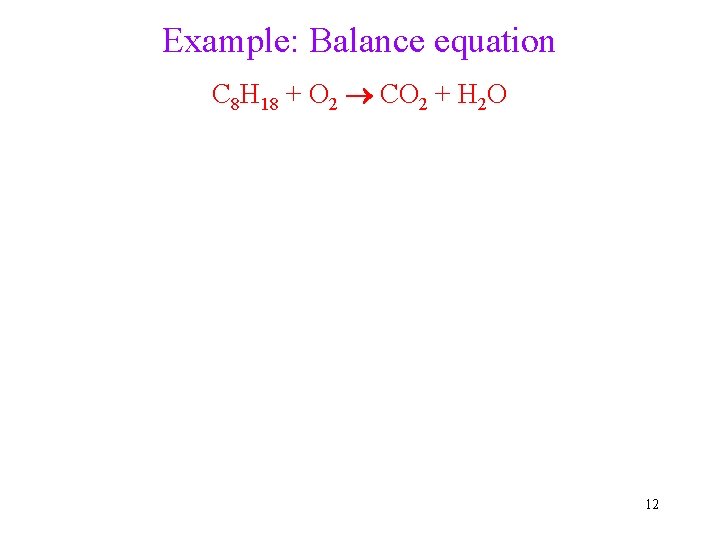
Example: Balance equation C 8 H 18 + O 2 CO 2 + H 2 O 12
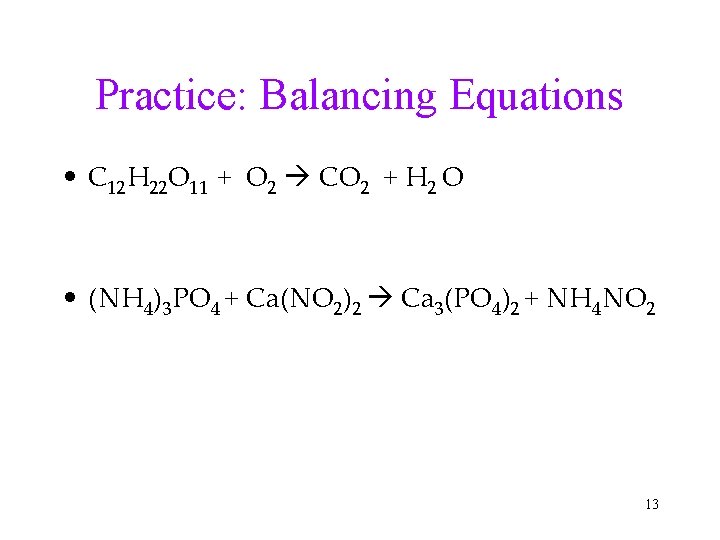
Practice: Balancing Equations • C 12 H 22 O 11 + O 2 CO 2 + H 2 O • (NH 4)3 PO 4 + Ca(NO 2)2 Ca 3(PO 4)2 + NH 4 NO 2 13
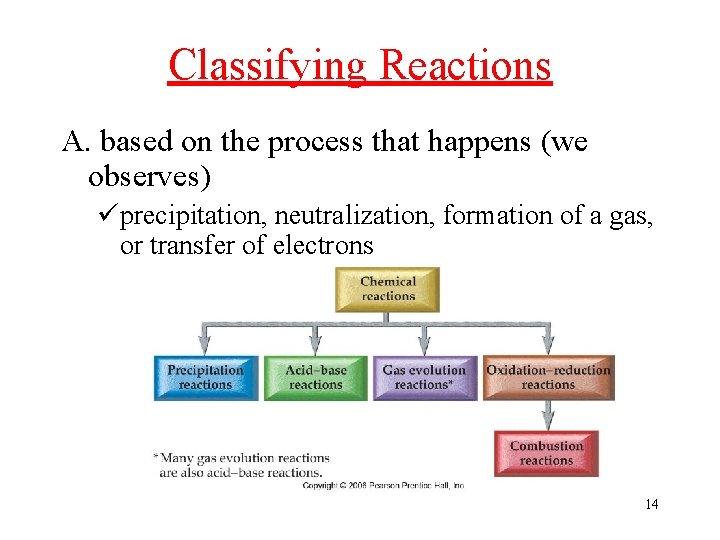
Classifying Reactions A. based on the process that happens (we observes) üprecipitation, neutralization, formation of a gas, or transfer of electrons 14
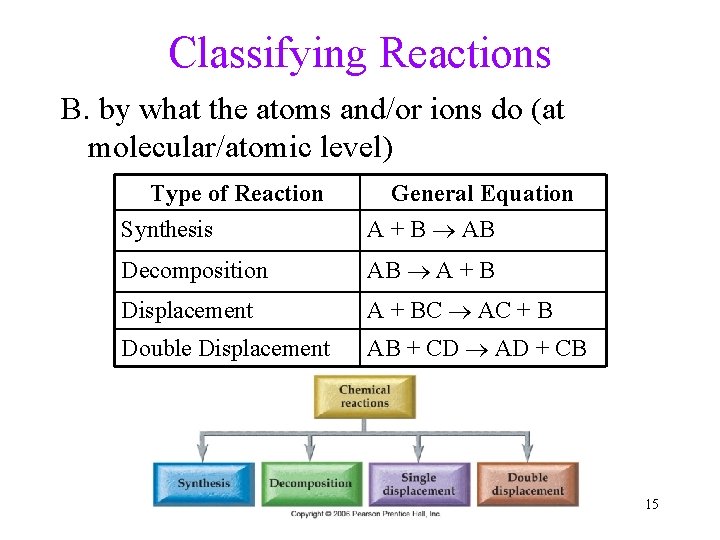
Classifying Reactions B. by what the atoms and/or ions do (at molecular/atomic level) Type of Reaction Synthesis General Equation A + B AB Decomposition AB A + B Displacement A + BC AC + B Double Displacement AB + CD AD + CB 15

Predicting Whether a Reaction Will Occur in Aqueous Solution “Forces” that drive a reaction 1) formation of a solid (aka “Precipitation”) 2) formation of water 3) formation of a gas 4) transfer of electrons • when chemicals (dissolved in water) are mixed and one of these 4 things can occur, the reaction will generally happen 16
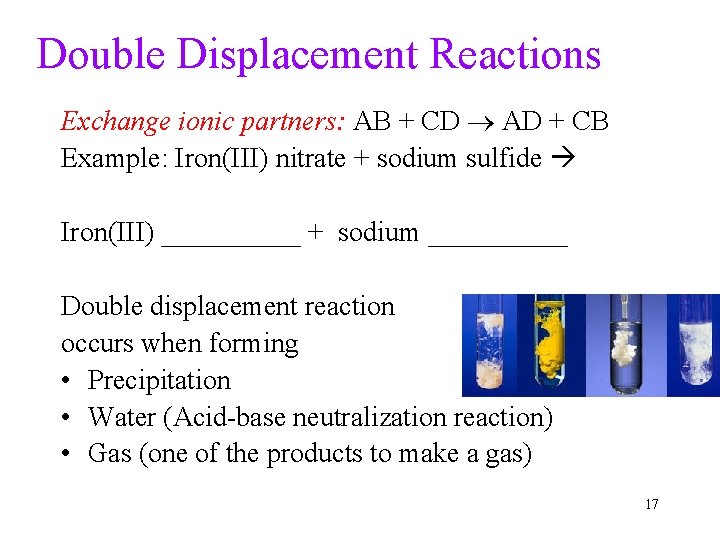
Double Displacement Reactions Exchange ionic partners: AB + CD AD + CB Example: Iron(III) nitrate + sodium sulfide Iron(III) _____ + sodium _____ Double displacement reaction occurs when forming • Precipitation • Water (Acid-base neutralization reaction) • Gas (one of the products to make a gas) 17

Reactions in Aqueous Solutions • Often the chemicals we are reacting together are dissolved in water ü Aqueous solutions = mixtures of a chemical dissolved in water • Dissolving the chemicals in water helps them to react together faster ü Water molecules separate the chemicals into individual molecules or ions ü The free floating particles collide more frequently so the reaction speeds up 18
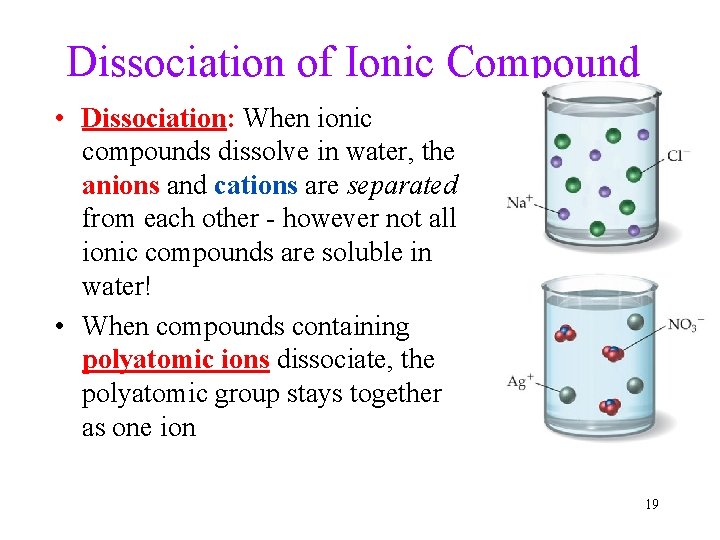
Dissociation of Ionic Compound • Dissociation: When ionic compounds dissolve in water, the anions and cations are separated from each other - however not all ionic compounds are soluble in water! • When compounds containing polyatomic ions dissociate, the polyatomic group stays together as one ion 19
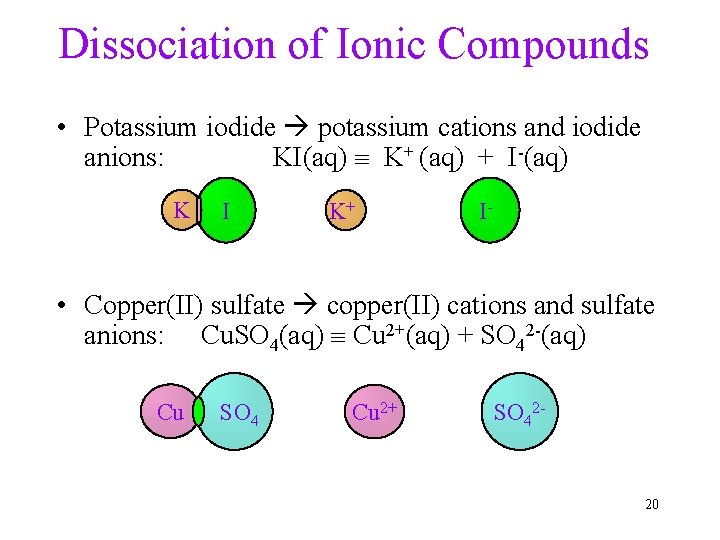
Dissociation of Ionic Compounds • Potassium iodide potassium cations and iodide anions: KI(aq) K+ (aq) + I-(aq) K I K+ I- • Copper(II) sulfate copper(II) cations and sulfate anions: Cu. SO 4(aq) Cu 2+(aq) + SO 42 -(aq) Cu SO 4 Cu 2+ SO 42 - 20
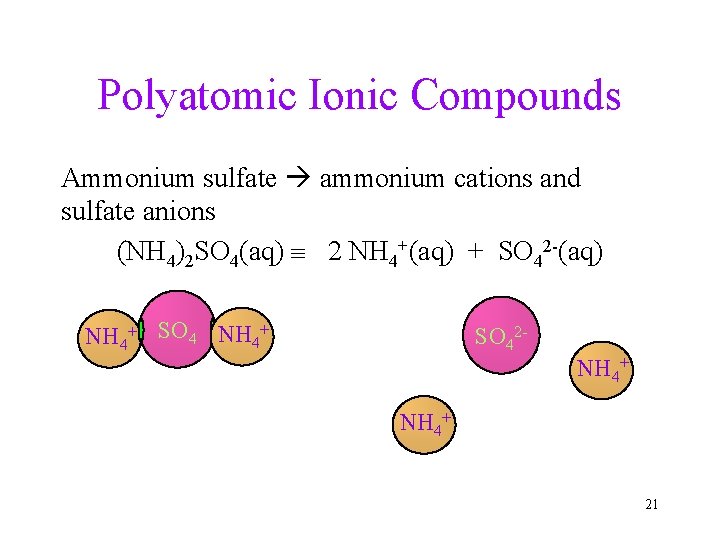
Polyatomic Ionic Compounds Ammonium sulfate ammonium cations and sulfate anions (NH 4)2 SO 4(aq) 2 NH 4+(aq) + SO 42 -(aq) NH 4+ SO 42 NH 4+ 21

Practice: How Ionic Compounds Dissociate? • Ammonium phosphate • Cobalt(III) sulfate • Zinc bromide 22
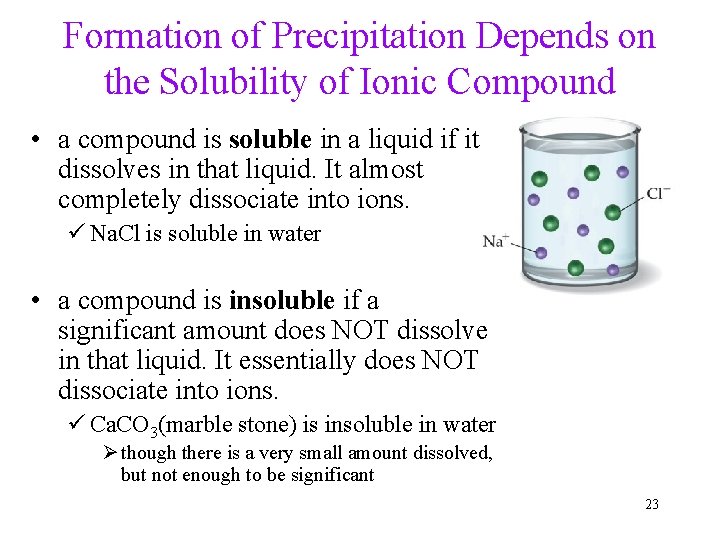
Formation of Precipitation Depends on the Solubility of Ionic Compound • a compound is soluble in a liquid if it dissolves in that liquid. It almost completely dissociate into ions. ü Na. Cl is soluble in water • a compound is insoluble if a significant amount does NOT dissolve in that liquid. It essentially does NOT dissociate into ions. ü Ca. CO 3(marble stone) is insoluble in water Ø though there is a very small amount dissolved, but not enough to be significant 23

How to Predict the Solubility of Ionic Compound? • Predicting whether a compound will dissolve in water is not easy Such knowledge comes through Experimentation: • Do some Experiments to test whether a compound will dissolve in water • Then develop some rules based on those experimental results ü we call this method the Empirical method 24
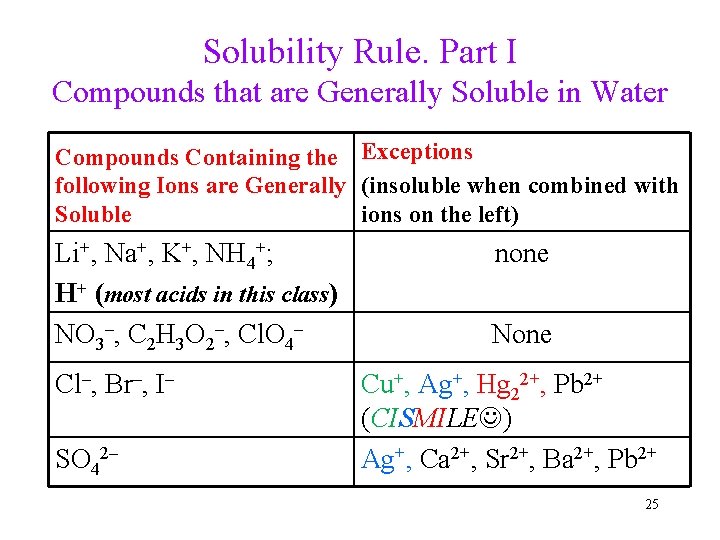
Solubility Rule. Part I Compounds that are Generally Soluble in Water Compounds Containing the Exceptions following Ions are Generally (insoluble when combined with Soluble ions on the left) Li+, Na+, K+, NH 4+; H+ (most acids in this class) NO 3–, C 2 H 3 O 2–, Cl. O 4– Cl–, Br–, I– SO 42– none None Cu+, Ag+, Hg 22+, Pb 2+ (CISMILE ) Ag+, Ca 2+, Sr 2+, Ba 2+, Pb 2+ 25
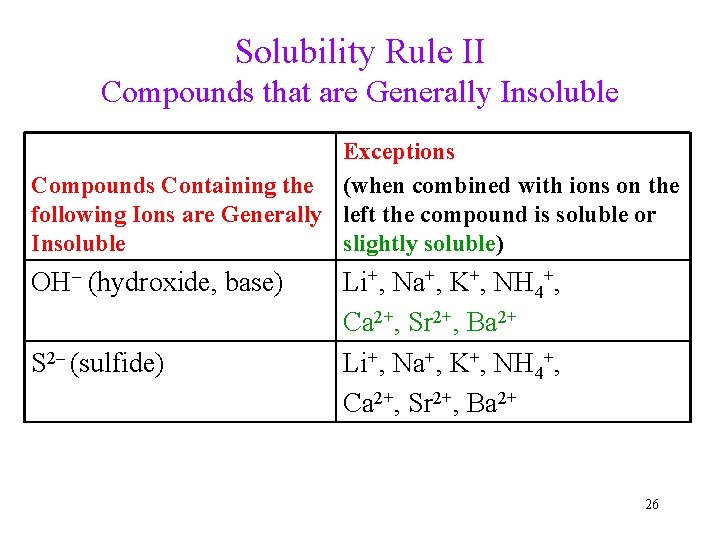
Solubility Rule II Compounds that are Generally Insoluble Exceptions Compounds Containing the (when combined with ions on the following Ions are Generally left the compound is soluble or Insoluble slightly soluble) OH– (hydroxide, base) S 2– (sulfide) Li+, Na+, K+, NH 4+, Ca 2+, Sr 2+, Ba 2+ 26
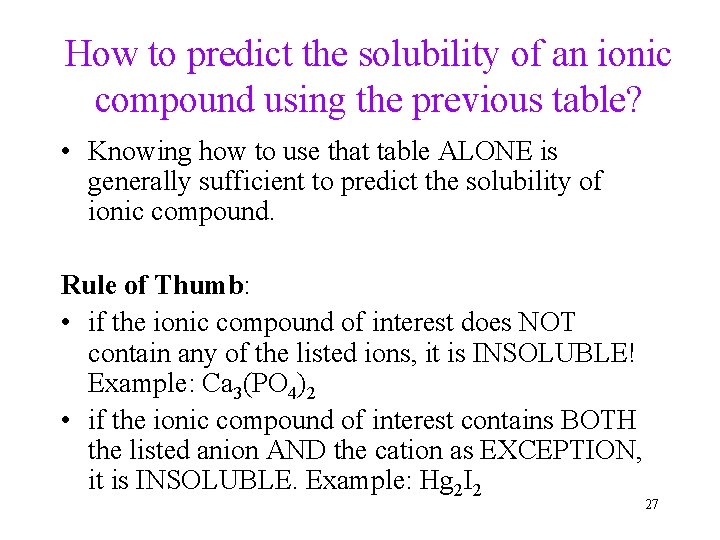
How to predict the solubility of an ionic compound using the previous table? • Knowing how to use that table ALONE is generally sufficient to predict the solubility of ionic compound. Rule of Thumb: • if the ionic compound of interest does NOT contain any of the listed ions, it is INSOLUBLE! Example: Ca 3(PO 4)2 • if the ionic compound of interest contains BOTH the listed anion AND the cation as EXCEPTION, it is INSOLUBLE. Example: Hg 2 I 2 27

Predict an Ionic Compound’s Solubility in Water: Hg. SO 4 1. Check the cation: Is it Li+, Na+, K+, or NH 4+? • If YES the compound will be soluble in water ü regardless of the anion! • If NO follow the rule for the anion (Table 7. 2) 2. if a rule says the compounds are mostly soluble, then the exceptions are insoluble 3. but if a rule says the compounds are mostly insoluble, then the exceptions are soluble 1. note: slightly soluble insoluble 28
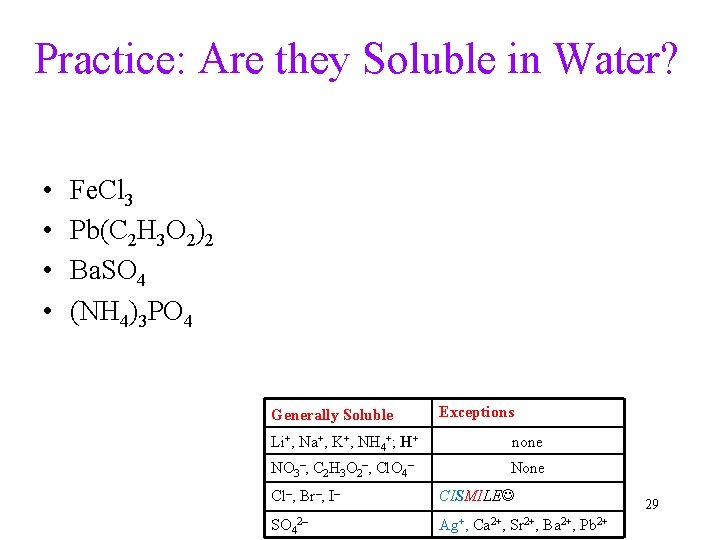
Practice: Are they Soluble in Water? • • Fe. Cl 3 Pb(C 2 H 3 O 2)2 Ba. SO 4 (NH 4)3 PO 4 Generally Soluble Exceptions Li+, Na+, K+, NH 4+; H+ none NO 3–, C 2 H 3 O 2–, Cl. O 4– None Cl–, Br–, I– CISMILE SO 42– Ag+, Ca 2+, Sr 2+, Ba 2+, Pb 2+ 29

Insoluble Ionic Compounds do not Dissociate into Ions • Insoluble Ionic compounds do not dissolve in water because the Coulombic force among the ions is too strong for water molecules to break down the compound. Therefore they do not dissociate into ions Ba. SO 4(s) Ba 2+(aq) + SO 42 -(aq) 30

Precipitation Reactions • When two soluble ionic compounds in aqueous solution mix, ion exchange will take place AB + CD AC + DB • if one of the products is insoluble in water, it will come out of solution as a precipitate 31
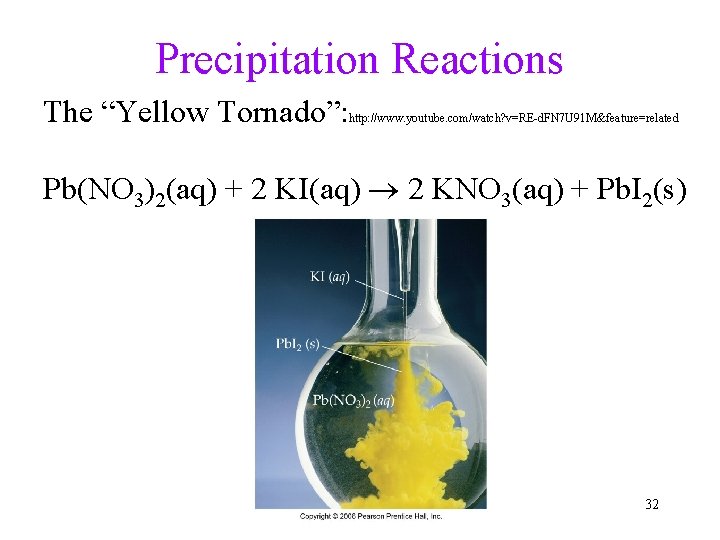
Precipitation Reactions The “Yellow Tornado”: http: //www. youtube. com/watch? v=RE-d. FN 7 U 91 M&feature=related Pb(NO 3)2(aq) + 2 KI(aq) 2 KNO 3(aq) + Pb. I 2(s) 32

No Precipitate Formation = No Reaction KI(aq) + Na. Cl(aq) KCl(aq) + Na. I(aq) all ions still present (all products are soluble), no reaction 33

Example: Write balanced equation between aqueous solution of sodium carbonate and aqueous solution of copper(II) chloride. Predict if precipitation forms. 1. 2. 4. 5. 6. 7. Write the formulas of the reactants Determine the ions present for each reactant Exchange the Ions Write the formulas of the products ü cross charges and reduce ü Balance the Equation Determine the solubility of each product Add “(s)” after the insoluble products and “(aq)” after the soluble products 34
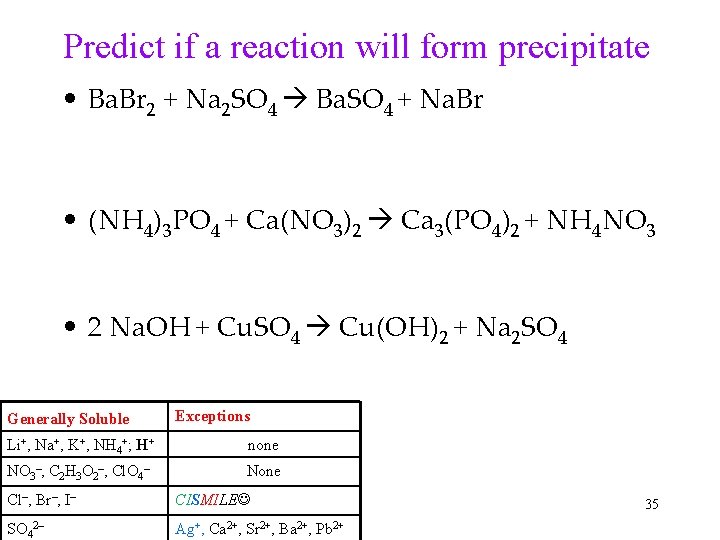
Predict if a reaction will form precipitate • Ba. Br 2 + Na 2 SO 4 Ba. SO 4 + Na. Br • (NH 4)3 PO 4 + Ca(NO 3)2 Ca 3(PO 4)2 + NH 4 NO 3 • 2 Na. OH + Cu. SO 4 Cu(OH)2 + Na 2 SO 4 Generally Soluble Exceptions Li+, Na+, K+, NH 4+; H+ none NO 3–, C 2 H 3 O 2–, Cl. O 4– None Cl–, Br–, I– CISMILE SO 42– Ag+, Ca 2+, Sr 2+, Ba 2+, Pb 2+ 35
Acid-Base Reactions Also as Neutralization reactions : the Acid and Base neutralize each other’s properties • H+ from the Acid + OH- from the base Water • Cation from the base + Anion from the acid salt (spectator ions) Acid + Base Salt + Water 2 HNO 3(aq) + Ca(OH)2(aq) Ca(NO 3)2(aq) + 2 H 2 O(l) 36

Example: - Write and Balance equation for the reaction of aqueous nitric acid with aqueous calcium hydroxide 1. 2. 3. 4. 5. 6. 7. Write the formulas of the reactants Determine the ions present for each reactant Exchange the ions, H+ combines with OH- to make H 2 O(l). Tip: write water as HOH Write the formulas of the products Balance the Equation (may be quickly balanced by matching the numbers of H and OH to make HOH) Determine the solubility of the salt Write an (s) after the insoluble products and a (aq) after the soluble products. Water exists as liquid, so (l) for water. 37
Gas-Evolving Reactions • Some reactions form a gas directly From the ion exchange K 2 S(aq) + H 2 SO 4(aq) K 2 SO 4(aq) + H 2 S(g) • Other reactions form a gas by the decomposition of one of the products into a gas and water Step 1: Na 2 CO 3(aq) + H 2 SO 4(aq) Na 2 SO 4(aq) + H 2 CO 3(aq) Step 2: H 2 CO 3 H 2 O(l) + CO 2(g) Overall reaction: Na 2 CO 3(aq) + H 2 SO 4(aq) Na 2 SO 4(aq) + H 2 O(l) + CO 2(g) 38
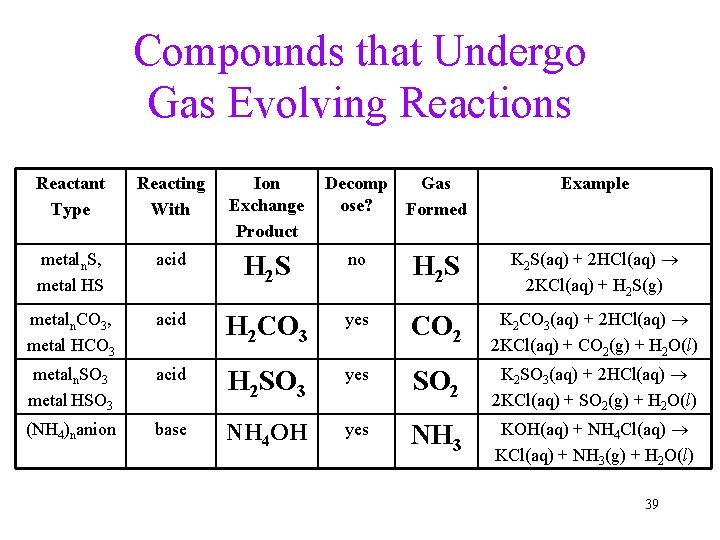
Compounds that Undergo Gas Evolving Reactions Reactant Type Reacting With Ion Exchange Product Decomp Gas ose? Formed Example metaln. S, metal HS acid H 2 S no H 2 S K 2 S(aq) + 2 HCl(aq) 2 KCl(aq) + H 2 S(g) metaln. CO 3, metal HCO 3 acid H 2 CO 3 yes CO 2 K 2 CO 3(aq) + 2 HCl(aq) 2 KCl(aq) + CO 2(g) + H 2 O(l) metaln. SO 3 metal HSO 3 acid H 2 SO 3 yes SO 2 K 2 SO 3(aq) + 2 HCl(aq) 2 KCl(aq) + SO 2(g) + H 2 O(l) (NH 4)nanion base NH 4 OH yes NH 3 KOH(aq) + NH 4 Cl(aq) KCl(aq) + NH 3(g) + H 2 O(l) 39
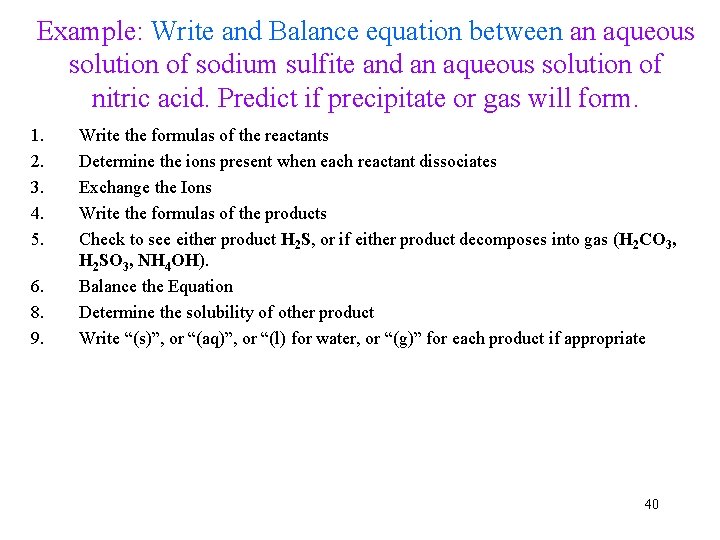
Example: Write and Balance equation between an aqueous solution of sodium sulfite and an aqueous solution of nitric acid. Predict if precipitate or gas will form. 1. 2. 3. 4. 5. 6. 8. 9. Write the formulas of the reactants Determine the ions present when each reactant dissociates Exchange the Ions Write the formulas of the products Check to see either product H 2 S, or if either product decomposes into gas (H 2 CO 3, H 2 SO 3, NH 4 OH). Balance the Equation Determine the solubility of other product Write “(s)”, or “(aq)”, or “(l) for water, or “(g)” for each product if appropriate 40
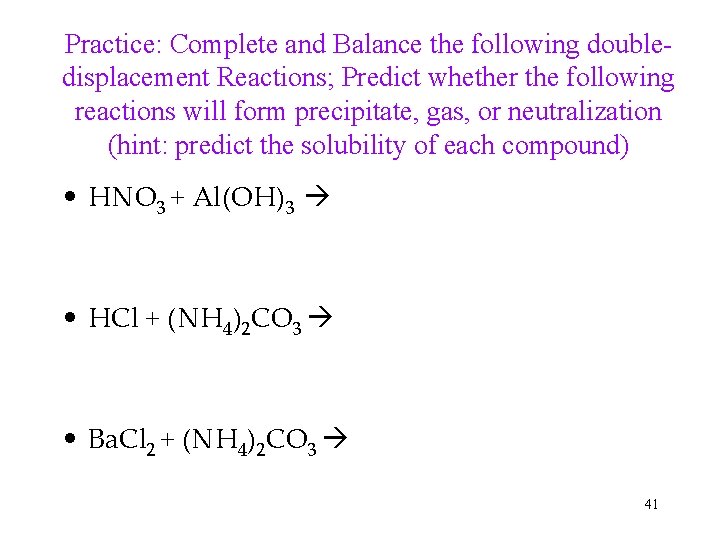
Practice: Complete and Balance the following doubledisplacement Reactions; Predict whether the following reactions will form precipitate, gas, or neutralization (hint: predict the solubility of each compound) • HNO 3 + Al(OH)3 • HCl + (NH 4)2 CO 3 • Ba. Cl 2 + (NH 4)2 CO 3 41
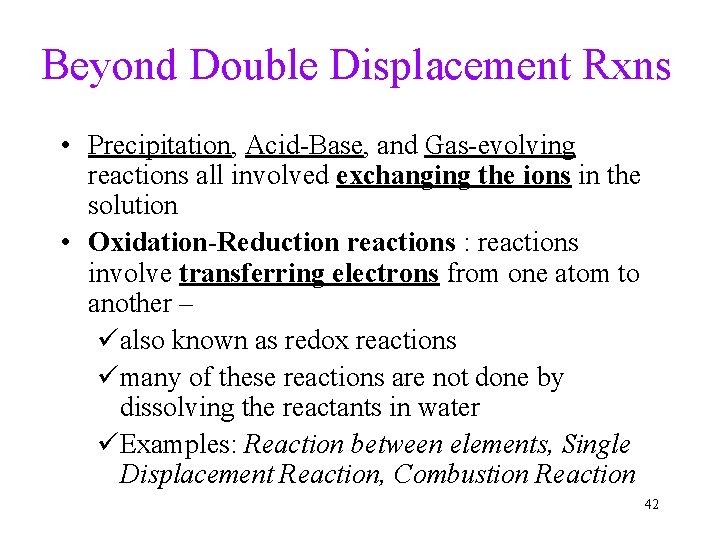
Beyond Double Displacement Rxns • Precipitation, Acid-Base, and Gas-evolving reactions all involved exchanging the ions in the solution • Oxidation-Reduction reactions : reactions involve transferring electrons from one atom to another – üalso known as redox reactions ümany of these reactions are not done by dissolving the reactants in water üExamples: Reaction between elements, Single Displacement Reaction, Combustion Reaction 42
Reactions of Metals with Nonmetals (Oxidation-Reduction) • metals react with nonmetals to form ionic compounds ü ionic compounds are solids at room temperature • the metal loses electrons and becomes a cation ü the metal undergoes oxidation: Na Na+ + e- • the nonmetal gains electrons and becomes an anion ü the nonmetal undergoes reduction: Cl 2+ 2 e- 2 Cl- Overall Reaction: 2 Na(s) + Cl 2(g) 2 Na. Cl(s) 43
Single-Replacement Reactions Aka Single-Displacement Reaction: A reaction in which a more active element displaces another less active element in a compound. A + BC AB + C • When iron bar placed in copper(II) sulfate solution: Iron is corroded, copper metal appears, blue color (Cu 2+ ion) fades. Fe(s) + Cu. SO 4(aq) → Fe. SO 4(aq) + Cu(s) Here ______ is more active than ____.
Aqueous Acid Displacements • Metals that precede (H) in the activity series react with acids, and those that follow (H) do not react with acids. • More active metals react with acid to produce hydrogen gas and an ionic compound. Fe(s) + 2 HCl(aq) → Fe. Cl 2(aq) + H 2(g) • Metals less active than (H) show no reaction. Au(s) + H 2 SO 4(aq) → NR

Active Metals and Water • A few metals are active enough to react directly with water. These are called active metals. • They react with water to produce a metal hydroxide and hydrogen gas. 2 Na(s) + 2 H 2 O(l) → 2 Na. OH(aq) + H 2(g) Ca(s) + 2 H 2 O(l) → Ca(OH)2(aq) + H 2(g)

Combustion Reactions • Reactions in which O 2(g) is a reactant are called Combustion Reactions • Combustion reactions release lots of energy • Combustion reactions are a subclass of Oxidation. Reduction reactions 2 C 8 H 18(g) + 25 O 2(g) 16 CO 2(g) + 18 H 2 O(g) 47
Combustion Products • to predict the products of a combustion reaction, combine each element in the other reactant with oxygen Reactant Combustion Product contains C CO 2(g) contains H H 2 O(g) contains S SO 2(g) contains N NO(g) or NO 2(g) contains metal M 2 On(s) Tro's Introductory Chemistry, Chapter 7 48
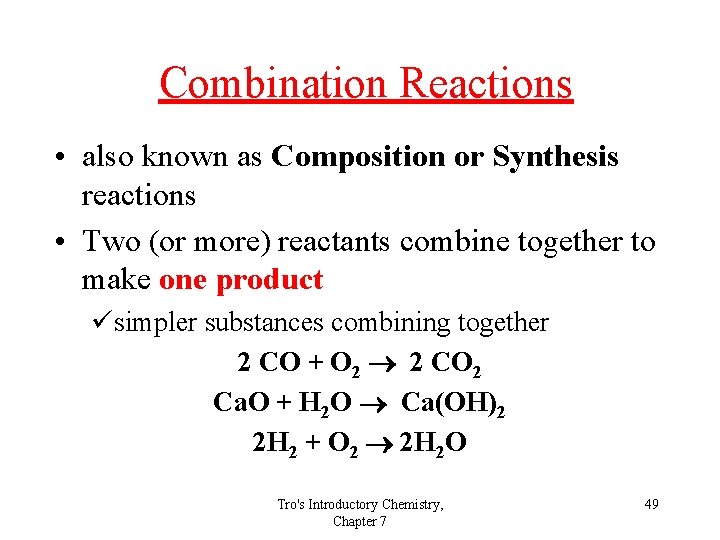
Combination Reactions • also known as Composition or Synthesis reactions • Two (or more) reactants combine together to make one product üsimpler substances combining together 2 CO + O 2 2 CO 2 Ca. O + H 2 O Ca(OH)2 2 H 2 + O 2 2 H 2 O Tro's Introductory Chemistry, Chapter 7 49
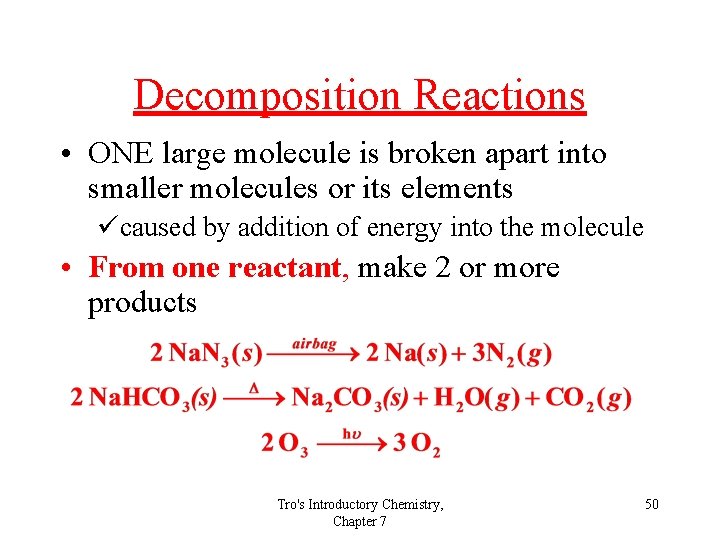
Decomposition Reactions • ONE large molecule is broken apart into smaller molecules or its elements ücaused by addition of energy into the molecule • From one reactant, make 2 or more products Tro's Introductory Chemistry, Chapter 7 50

Practice: Write and Balance Word Equations • iron(III) chloride + copper metal iron(II) chloride + copper(II) chloride • nitrogen gas + magnesium metal magnesium nitride • zinc phosphate + hydrochloric acid phosphoric acid + zinc chloride 51

Determine if Each of the Following is Soluble in Water • KOH Soluble, because the cation is K+ • Ag. Br Insoluble, even though most compounds with Br- are soluble, this is an exception • Ca. Cl 2 Soluble, most compounds with Cl- are soluble • Pb(NO 3)2 Soluble, because the anion is NO 3 • Sr. SO 4 Insoluble, even though most compounds with SO 42 - are soluble, this is an exception 52
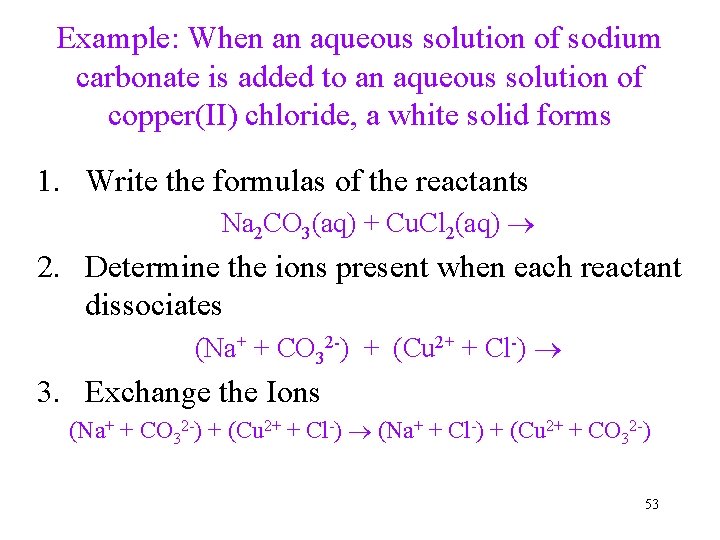
Example: When an aqueous solution of sodium carbonate is added to an aqueous solution of copper(II) chloride, a white solid forms 1. Write the formulas of the reactants Na 2 CO 3(aq) + Cu. Cl 2(aq) 2. Determine the ions present when each reactant dissociates (Na+ + CO 32 -) + (Cu 2+ + Cl-) 3. Exchange the Ions (Na+ + CO 32 -) + (Cu 2+ + Cl-) (Na+ + Cl-) + (Cu 2+ + CO 32 -) 53

Example: When an aqueous solution of sodium carbonate is added to an aqueous solution of copper(II) chloride, a white solid forms 4. Write the formulas of the products ü cross charges and reduce Na 2 CO 3(aq) + Cu. Cl 2(aq) Na. Cl + Cu. CO 3 5. Balance the Equation Na 2 CO 3(aq) + Cu. Cl 2(aq) 2 Na. Cl + Cu. CO 3 54
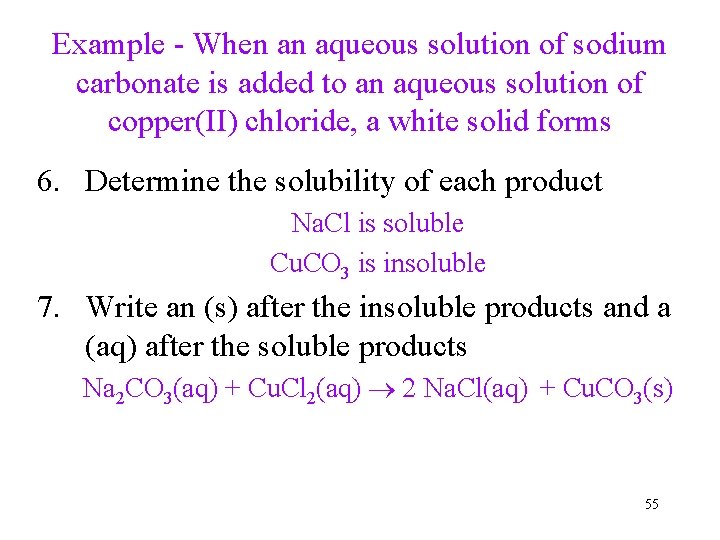
Example - When an aqueous solution of sodium carbonate is added to an aqueous solution of copper(II) chloride, a white solid forms 6. Determine the solubility of each product Na. Cl is soluble Cu. CO 3 is insoluble 7. Write an (s) after the insoluble products and a (aq) after the soluble products Na 2 CO 3(aq) + Cu. Cl 2(aq) 2 Na. Cl(aq) + Cu. CO 3(s) 55
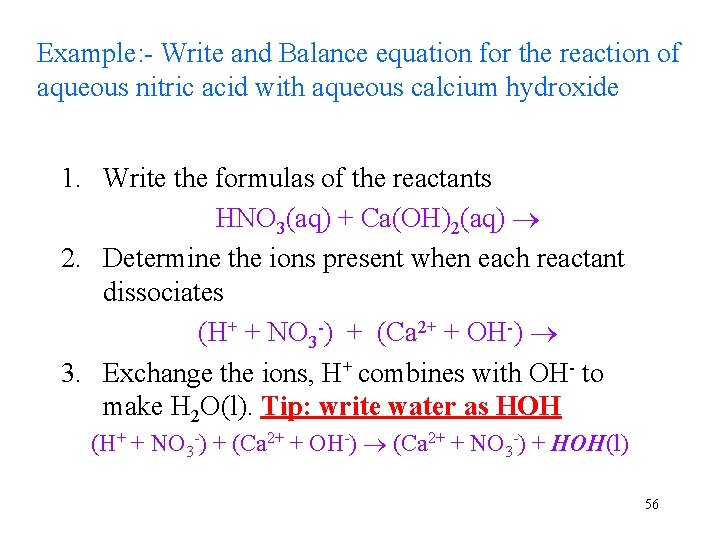
Example: - Write and Balance equation for the reaction of aqueous nitric acid with aqueous calcium hydroxide 1. Write the formulas of the reactants HNO 3(aq) + Ca(OH)2(aq) 2. Determine the ions present when each reactant dissociates (H+ + NO 3 -) + (Ca 2+ + OH-) 3. Exchange the ions, H+ combines with OH- to make H 2 O(l). Tip: write water as HOH (H+ + NO 3 -) + (Ca 2+ + OH-) (Ca 2+ + NO 3 -) + HOH(l) 56
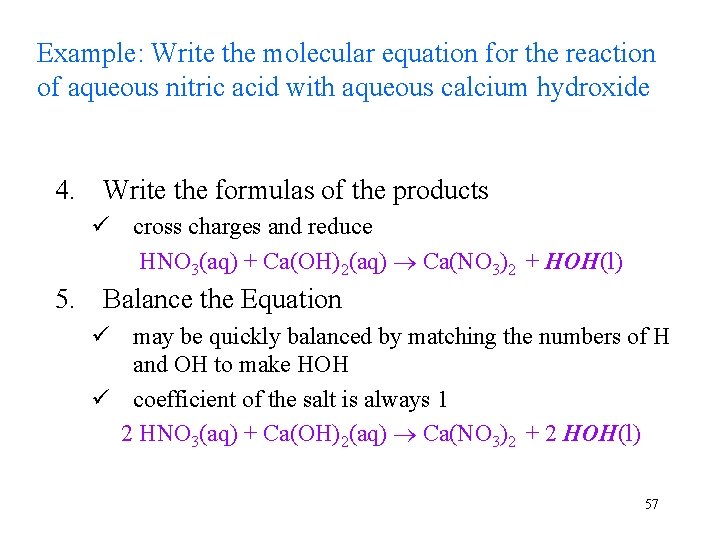
Example: Write the molecular equation for the reaction of aqueous nitric acid with aqueous calcium hydroxide 4. Write the formulas of the products ü cross charges and reduce HNO 3(aq) + Ca(OH)2(aq) Ca(NO 3)2 + HOH(l) 5. Balance the Equation ü may be quickly balanced by matching the numbers of H and OH to make HOH ü coefficient of the salt is always 1 2 HNO 3(aq) + Ca(OH)2(aq) Ca(NO 3)2 + 2 HOH(l) 57
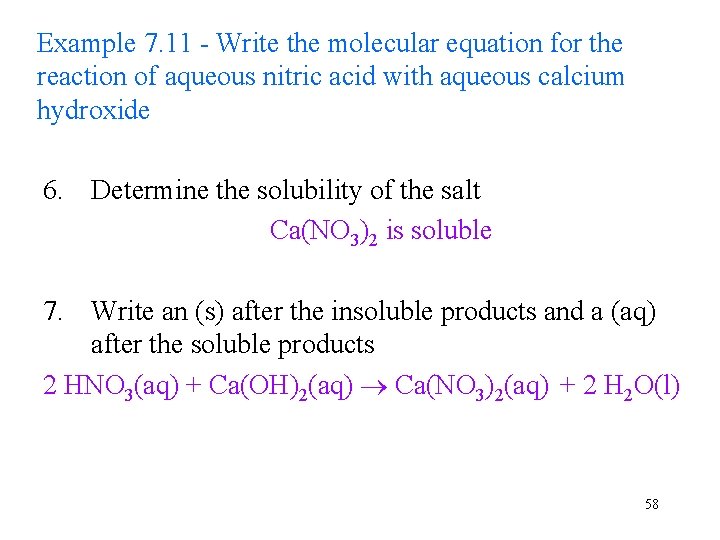
Example 7. 11 - Write the molecular equation for the reaction of aqueous nitric acid with aqueous calcium hydroxide 6. Determine the solubility of the salt Ca(NO 3)2 is soluble 7. Write an (s) after the insoluble products and a (aq) after the soluble products 2 HNO 3(aq) + Ca(OH)2(aq) Ca(NO 3)2(aq) + 2 H 2 O(l) 58
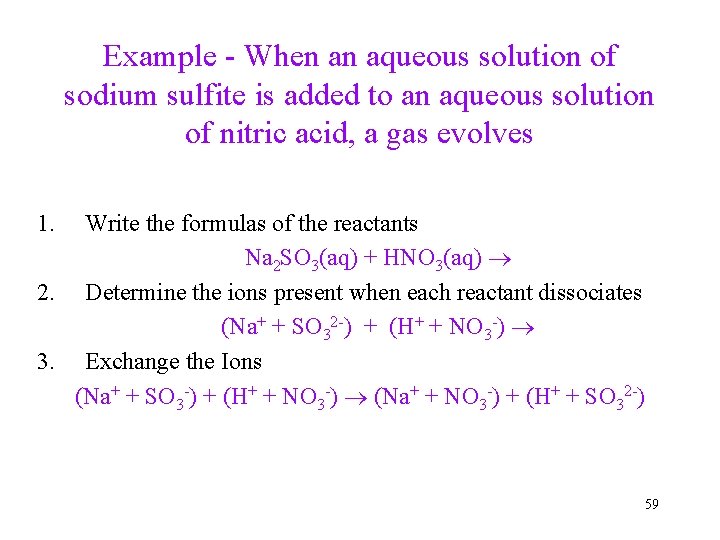
Example - When an aqueous solution of sodium sulfite is added to an aqueous solution of nitric acid, a gas evolves 1. Write the formulas of the reactants Na 2 SO 3(aq) + HNO 3(aq) 2. Determine the ions present when each reactant dissociates (Na+ + SO 32 -) + (H+ + NO 3 -) 3. Exchange the Ions (Na+ + SO 3 -) + (H+ + NO 3 -) (Na+ + NO 3 -) + (H+ + SO 32 -) 59
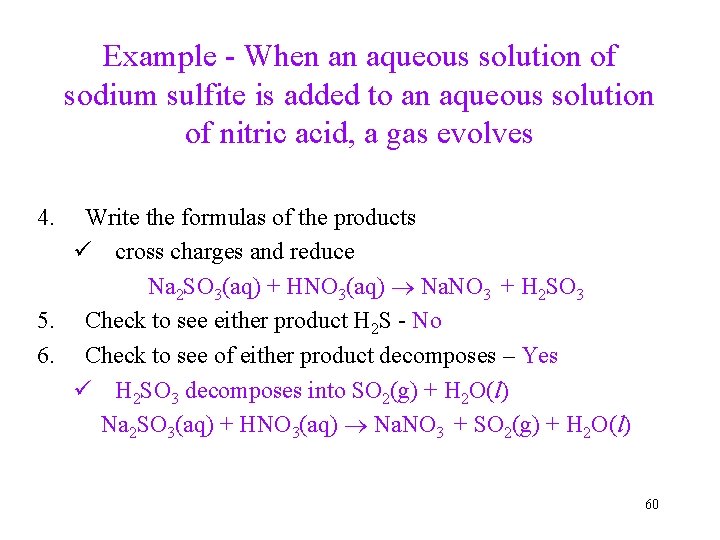
Example - When an aqueous solution of sodium sulfite is added to an aqueous solution of nitric acid, a gas evolves 4. Write the formulas of the products ü cross charges and reduce Na 2 SO 3(aq) + HNO 3(aq) Na. NO 3 + H 2 SO 3 5. Check to see either product H 2 S - No 6. Check to see of either product decomposes – Yes ü H 2 SO 3 decomposes into SO 2(g) + H 2 O(l) Na 2 SO 3(aq) + HNO 3(aq) Na. NO 3 + SO 2(g) + H 2 O(l) 60
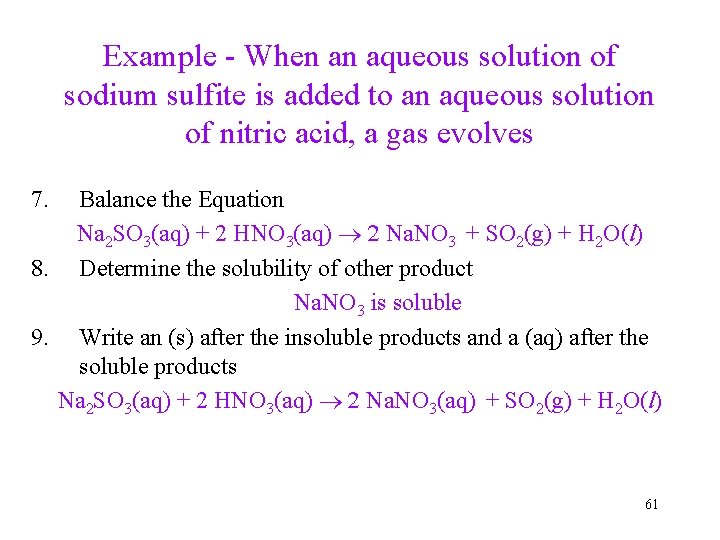
Example - When an aqueous solution of sodium sulfite is added to an aqueous solution of nitric acid, a gas evolves 7. Balance the Equation Na 2 SO 3(aq) + 2 HNO 3(aq) 2 Na. NO 3 + SO 2(g) + H 2 O(l) 8. Determine the solubility of other product Na. NO 3 is soluble 9. Write an (s) after the insoluble products and a (aq) after the soluble products Na 2 SO 3(aq) + 2 HNO 3(aq) 2 Na. NO 3(aq) + SO 2(g) + H 2 O(l) 61

Practice: Complete and Balance the following Word equations • copper(II) nitrate + sodium carbonate • silver acetate + ammonium chloride • potassium carbonate + phosphoric acid • barium hydroxide + sulfuric acid 62

Practice: Complete and Balance the following Word equations • copper(II) nitrate + sodium carbonate Cu(NO 3)2(aq) + Na 2 CO 3(aq) 2 Na. NO 3(aq) + Cu. CO 3(s) • silver acetate + ammonium chloride Ag. C 2 H 3 O 2(aq) + NH 4 Cl(aq) Ag. Cl(s) + NH 4 C 2 H 3 O 2(aq) • potassium carbonate + phosphoric acid • barium hydroxide + sulfuric acid 3 K 2 CO 3(aq) + 2 H 3 PO 4(aq) 2 K 3 PO 4(aq) + 3 H 2 O(l) + 3 CO 2(g) Ba(OH)2(aq) + H 2 SO 4(aq) Ba. SO 4(s) + 2 H 2 O(l) 63
- Slides: 63