Chemical Reactions and Quantities The Mole General Organic





- Slides: 20

Chemical Reactions and Quantities The Mole General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

Chemical Reactions and Quantities Vocabulary Mole Avogadro’s Number Formula Unit Conversion Factor Atomic Mass Unit (amu) Molar Mass General, Organic, and Biological Chemistry Review Vocabulary Atom Molecule Ionic Compound Covalent Compound Particle Copyright © 2010 Pearson Education, Inc. 2

The Mole Definitions in English 1. A small congenital growth on the human skin, usually slightly raised and dark and sometimes hairy 2. Any of various small, insect-eating mammals, esp. of the family Talpidae, living chiefly underground and having velvety fur, very small eyes, and strong forefeet. 3. A spy who becomes part of and works from within the ranks of an enemy governmental staff or intelligence agency. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

Chemical Reactions and Quantities Definitions in Chemistry 1. The amount of a substance that contains as many atoms, molecules, ions, or other elementary units as the number of atoms in 0. 012 kilogram of carbon 12. The number is 6. 0225 × 1023, or Avogadro's number. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

Collection Terms A collection term states a specific number of items. § 1 dozen donuts = 12 donuts § 1 ream of paper = 500 sheets § 1 case = 24 cans General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5

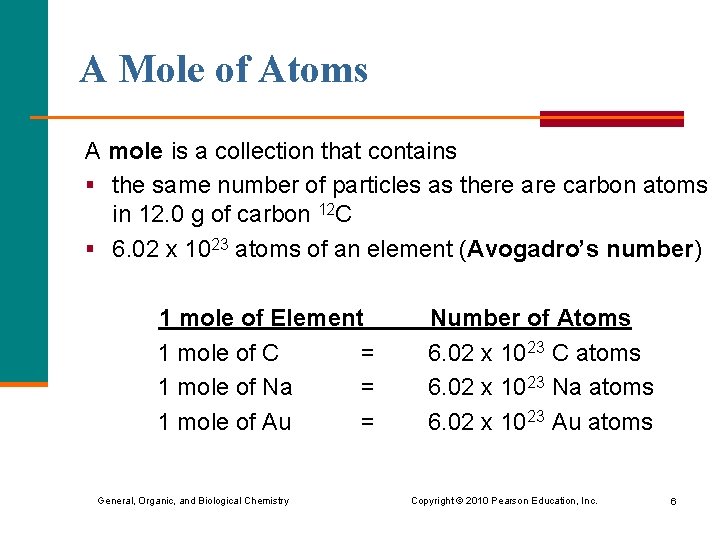
A Mole of Atoms A mole is a collection that contains § the same number of particles as there are carbon atoms in 12. 0 g of carbon 12 C § 6. 02 x 1023 atoms of an element (Avogadro’s number) 1 mole of Element 1 mole of C = 1 mole of Na = 1 mole of Au = General, Organic, and Biological Chemistry Number of Atoms 6. 02 x 1023 C atoms 6. 02 x 1023 Na atoms 6. 02 x 1023 Au atoms Copyright © 2010 Pearson Education, Inc. 6

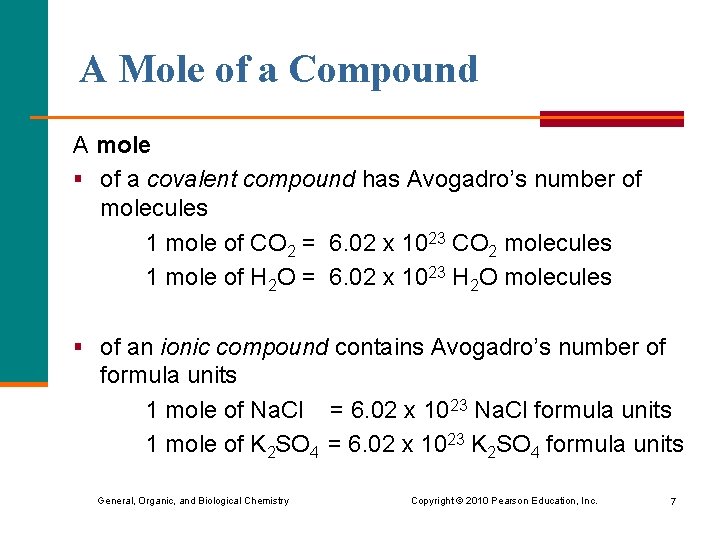
A Mole of a Compound A mole § of a covalent compound has Avogadro’s number of molecules 1 mole of CO 2 = 6. 02 x 1023 CO 2 molecules 1 mole of H 2 O = 6. 02 x 1023 H 2 O molecules § of an ionic compound contains Avogadro’s number of formula units 1 mole of Na. Cl = 6. 02 x 1023 Na. Cl formula units 1 mole of K 2 SO 4 = 6. 02 x 1023 K 2 SO 4 formula units General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7

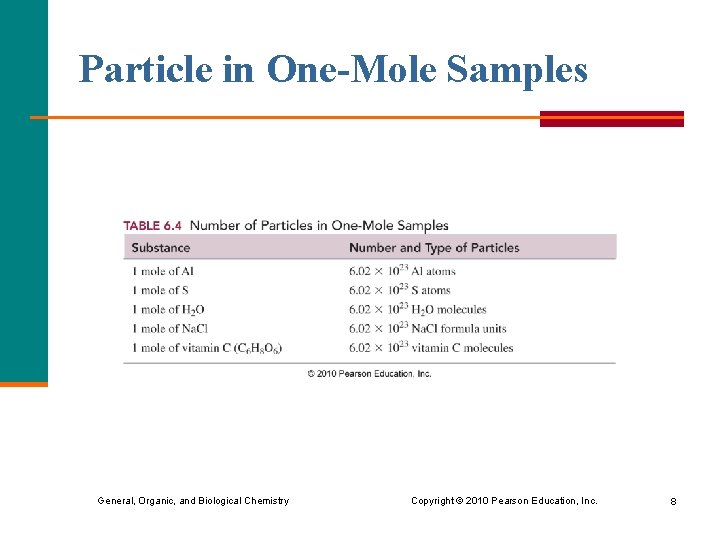
Particle in One-Mole Samples General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

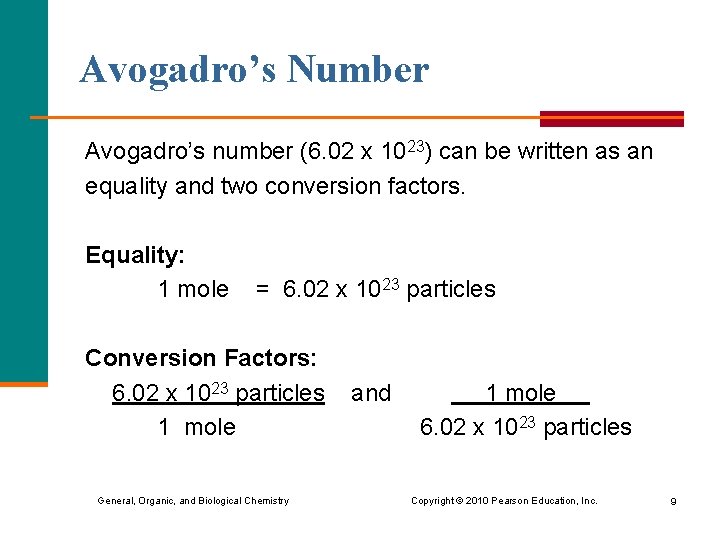
Avogadro’s Number Avogadro’s number (6. 02 x 1023) can be written as an equality and two conversion factors. Equality: 1 mole = 6. 02 x 1023 particles Conversion Factors: 6. 02 x 1023 particles 1 mole General, Organic, and Biological Chemistry and 1 mole 6. 02 x 1023 particles Copyright © 2010 Pearson Education, Inc. 9

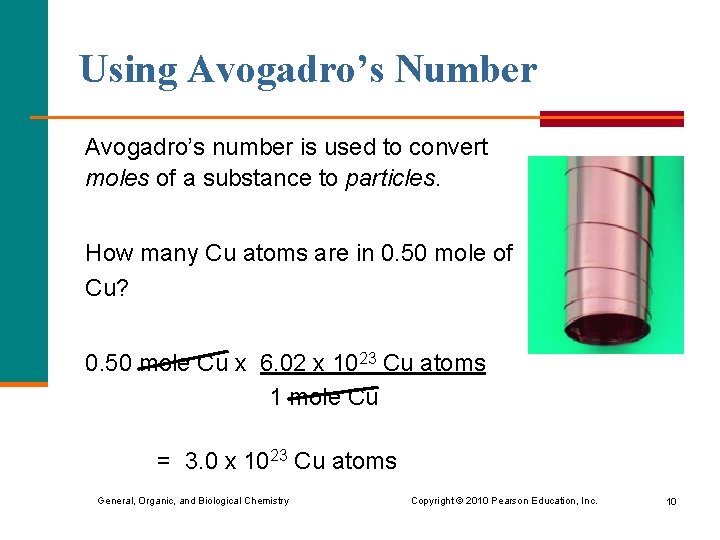
Using Avogadro’s Number Avogadro’s number is used to convert moles of a substance to particles. How many Cu atoms are in 0. 50 mole of Cu? 0. 50 mole Cu x 6. 02 x 1023 Cu atoms 1 mole Cu = 3. 0 x 1023 Cu atoms General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10

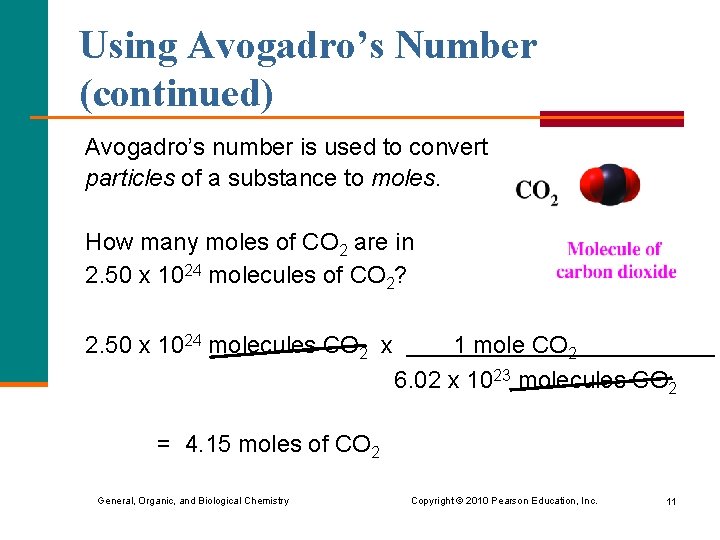
Using Avogadro’s Number (continued) Avogadro’s number is used to convert particles of a substance to moles. How many moles of CO 2 are in 2. 50 x 1024 molecules of CO 2? 2. 50 x 1024 molecules CO 2 x 1 mole CO 2 6. 02 x 1023 molecules CO 2 = 4. 15 moles of CO 2 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11

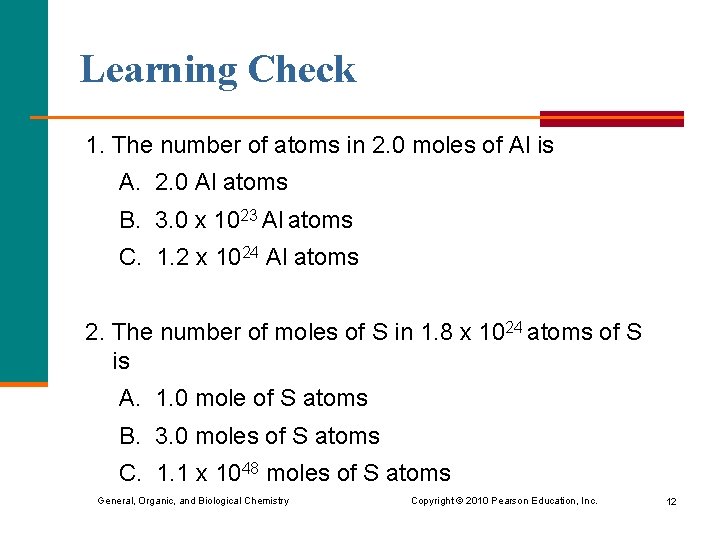
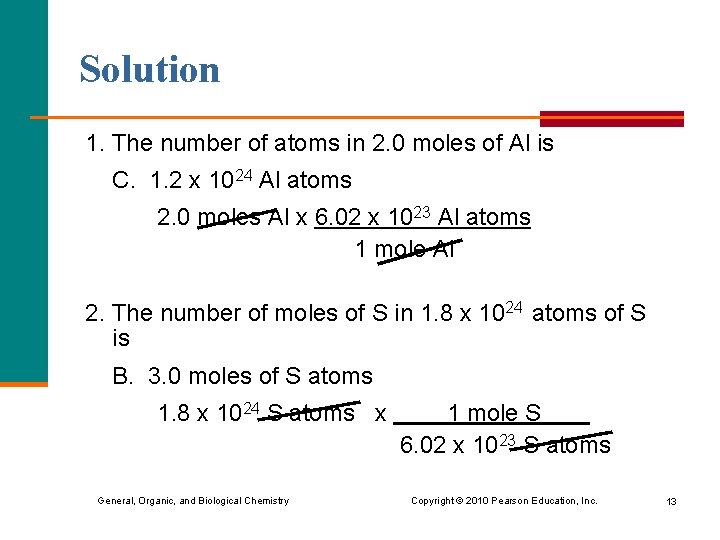
Learning Check 1. The number of atoms in 2. 0 moles of Al is A. 2. 0 Al atoms B. 3. 0 x 1023 Al atoms C. 1. 2 x 1024 Al atoms 2. The number of moles of S in 1. 8 x 1024 atoms of S is A. 1. 0 mole of S atoms B. 3. 0 moles of S atoms C. 1. 1 x 1048 moles of S atoms General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12

Solution 1. The number of atoms in 2. 0 moles of Al is C. 1. 2 x 1024 Al atoms 2. 0 moles Al x 6. 02 x 1023 Al atoms 1 mole Al 2. The number of moles of S in 1. 8 x 1024 atoms of S is B. 3. 0 moles of S atoms 1. 8 x 1024 S atoms x General, Organic, and Biological Chemistry 1 mole S 6. 02 x 1023 S atoms Copyright © 2010 Pearson Education, Inc. 13

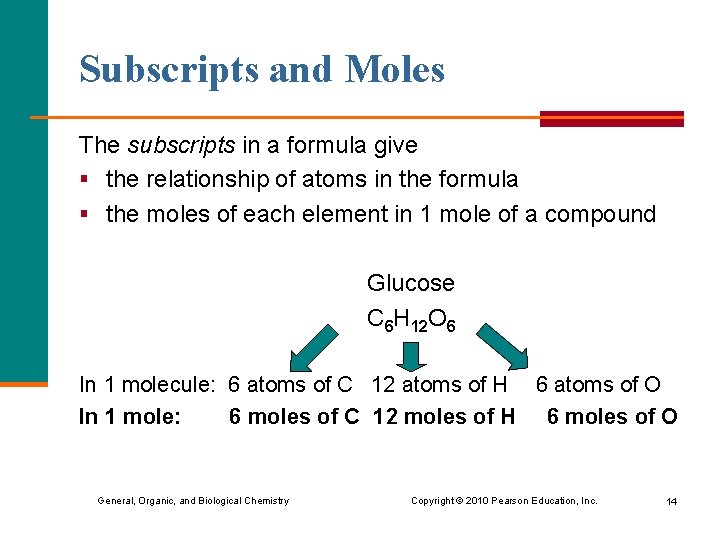
Subscripts and Moles The subscripts in a formula give § the relationship of atoms in the formula § the moles of each element in 1 mole of a compound Glucose C 6 H 12 O 6 In 1 molecule: 6 atoms of C 12 atoms of H 6 atoms of O In 1 mole: 6 moles of C 12 moles of H 6 moles of O General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 14

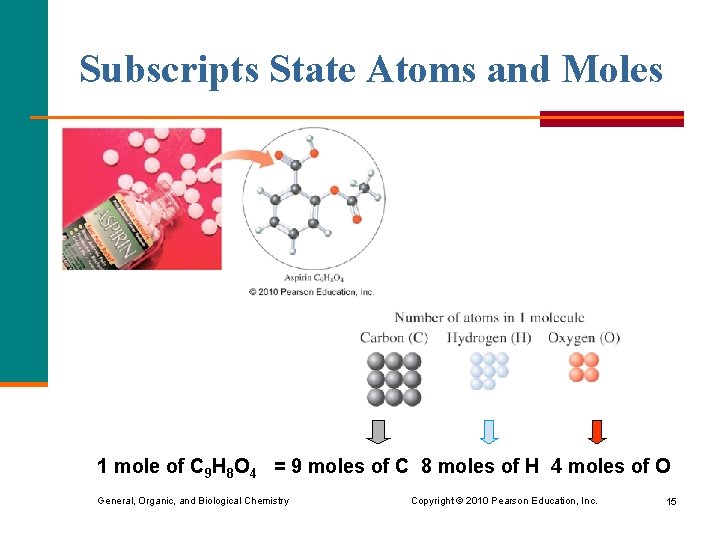
Subscripts State Atoms and Moles 1 mole of C 9 H 8 O 4 = 9 moles of C 8 moles of H 4 moles of O General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 15

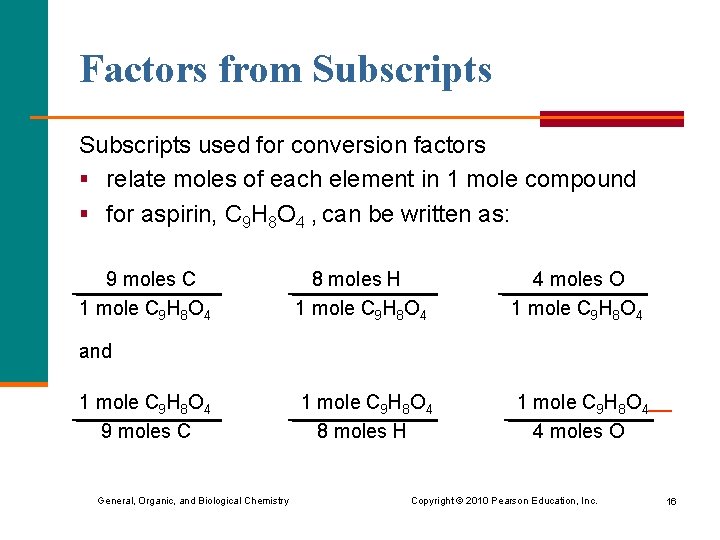
Factors from Subscripts used for conversion factors § relate moles of each element in 1 mole compound § for aspirin, C 9 H 8 O 4 , can be written as: 9 moles C 1 mole C 9 H 8 O 4 8 moles H 1 mole C 9 H 8 O 4 4 moles O and 1 mole C 9 H 8 O 4 9 moles C General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 16

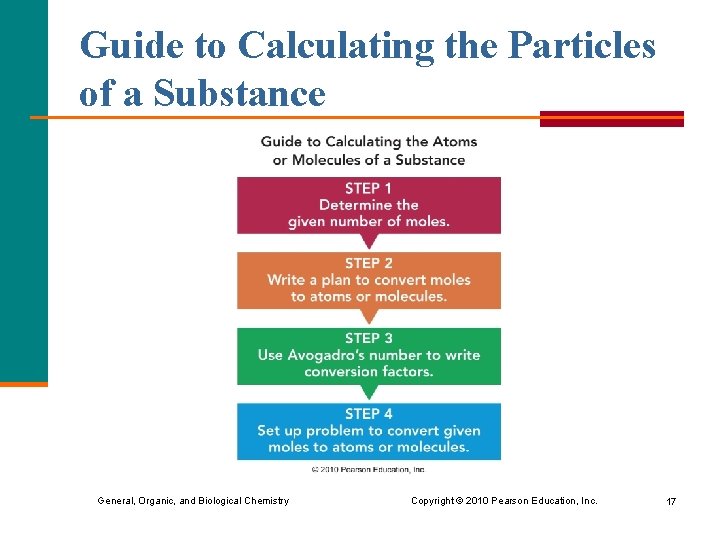
Guide to Calculating the Particles of a Substance General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 17

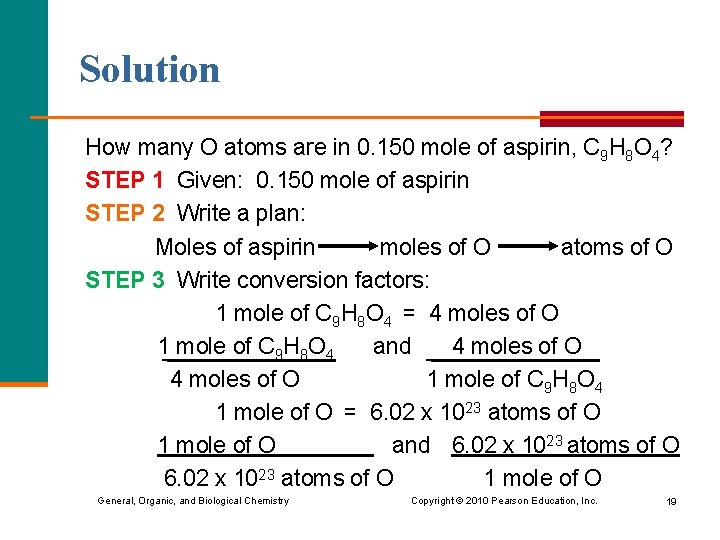
Learning Check How many O atoms are in 0. 150 mole of aspirin, C 9 H 8 O 4? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 18

Solution How many O atoms are in 0. 150 mole of aspirin, C 9 H 8 O 4? STEP 1 Given: 0. 150 mole of aspirin STEP 2 Write a plan: Moles of aspirin moles of O atoms of O STEP 3 Write conversion factors: 1 mole of C 9 H 8 O 4 = 4 moles of O 1 mole of C 9 H 8 O 4 and 4 moles of O 1 mole of C 9 H 8 O 4 1 mole of O = 6. 02 x 1023 atoms of O 1 mole of O and 6. 02 x 1023 atoms of O 1 mole of O General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 19

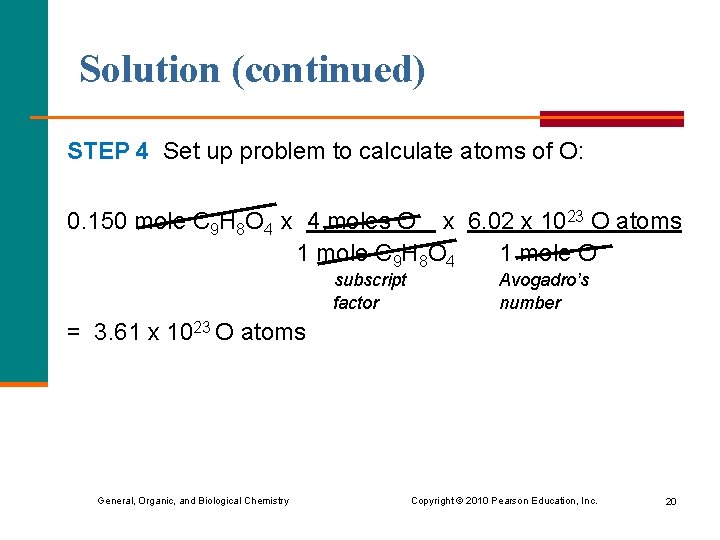
Solution (continued) STEP 4 Set up problem to calculate atoms of O: 0. 150 mole C 9 H 8 O 4 x 4 moles O x 6. 02 x 1023 O atoms 1 mole C 9 H 8 O 4 1 mole O subscript factor Avogadro’s number = 3. 61 x 1023 O atoms General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 20