Chemical Reactions And How to Balance Chemical Equations

Chemical Reactions And How to Balance Chemical Equations

Objectives 1. Know the five types of chemical reactions. 2. Be able to predict the products of a reaction. 3. Balance chemical reactions.
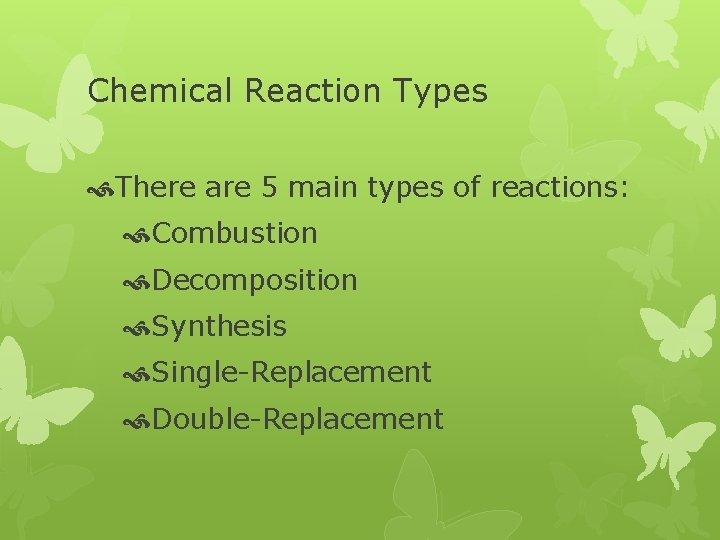
Chemical Reaction Types There are 5 main types of reactions: Combustion Decomposition Synthesis Single-Replacement Double-Replacement
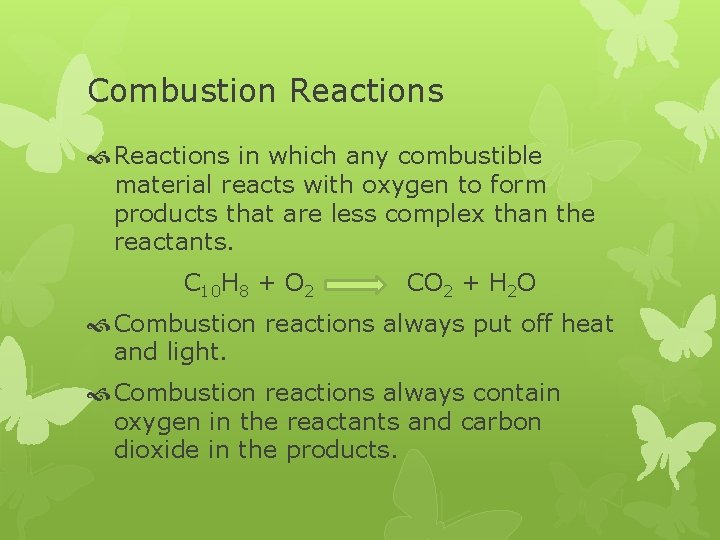
Combustion Reactions in which any combustible material reacts with oxygen to form products that are less complex than the reactants. C 10 H 8 + O 2 CO 2 + H 2 O Combustion reactions always put off heat and light. Combustion reactions always contain oxygen in the reactants and carbon dioxide in the products.

Decomposition Reactions A complex substance breaks down into two or more simpler substances. XY X+Y Example: When heated, potassium chlorate decomposes into oxygen and potassium chloride. KCl. O 3 KCl + O 2

Decomposition Reactions Heating sodium bicarbonate (baking soda) releases water, carbon dioxide, and sodium carbonate. Na. HCO 3 Na 2 CO 3 + H 2 O + CO 2 Handy when baking
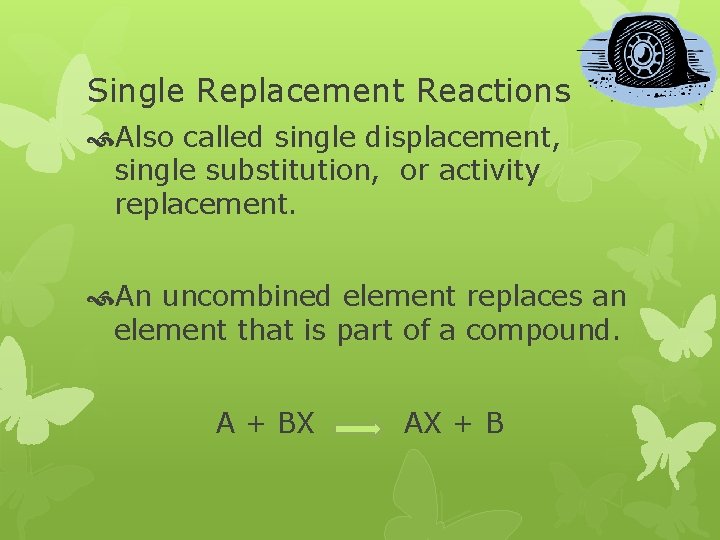
Single Replacement Reactions Also called single displacement, single substitution, or activity replacement. An uncombined element replaces an element that is part of a compound. A + BX AX + B

Single Replacement Examples Purifying Silver: Ag. NO 3 + Cu Cu(NO 3)2 + Ag Liberating Hydrogen: Zn + HCl Zn. Cl 2 + H 2 Notice that one element replaces another element. In both cases above an element is removed from the compound.
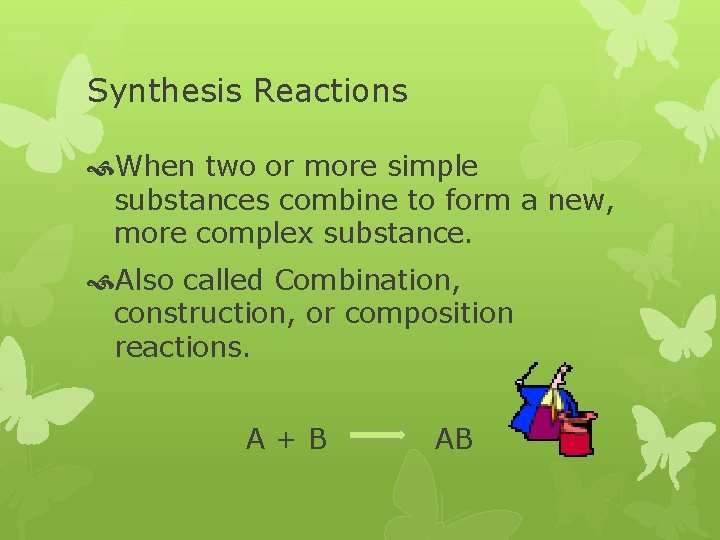
Synthesis Reactions When two or more simple substances combine to form a new, more complex substance. Also called Combination, construction, or composition reactions. A+B AB
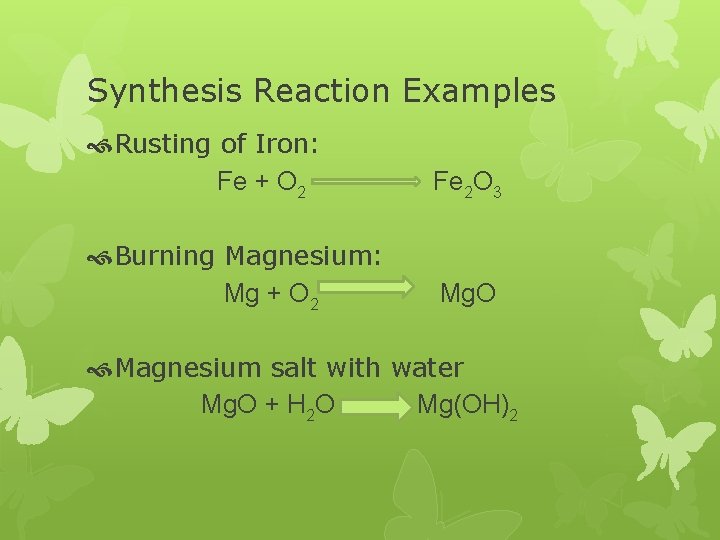
Synthesis Reaction Examples Rusting of Iron: Fe + O 2 Fe 2 O 3 Burning Magnesium: Mg + O 2 Mg. O Magnesium salt with water Mg. O + H 2 O Mg(OH)2

Double Replacement Reactions Also called double displacement or Metathesis Different atoms in two different compounds replace each other. AX + BY AY + BX
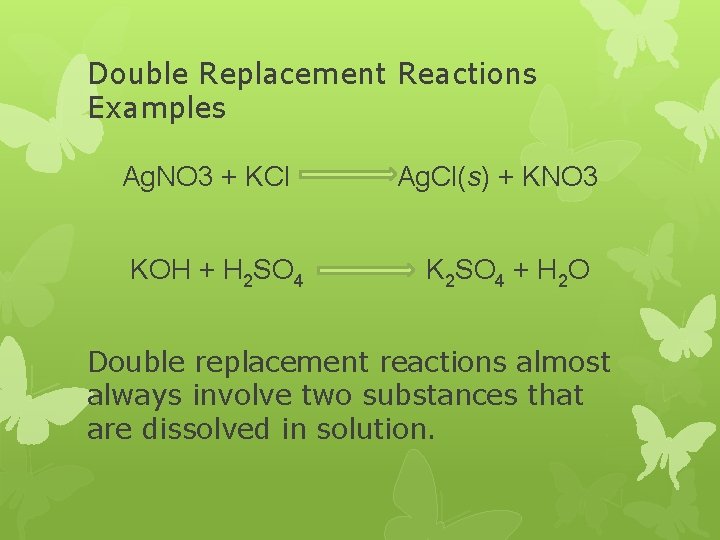
Double Replacement Reactions Examples Ag. NO 3 + KCl KOH + H 2 SO 4 Ag. Cl(s) + KNO 3 K 2 SO 4 + H 2 O Double replacement reactions almost always involve two substances that are dissolved in solution.
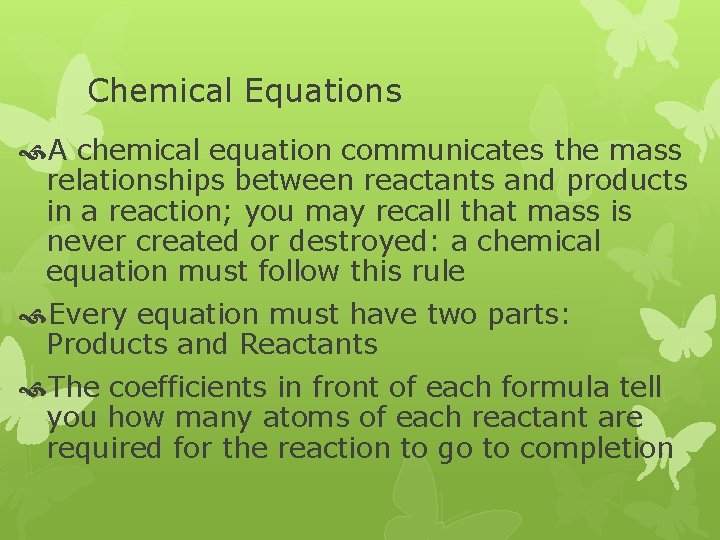
Chemical Equations A chemical equation communicates the mass relationships between reactants and products in a reaction; you may recall that mass is never created or destroyed: a chemical equation must follow this rule Every equation must have two parts: Products and Reactants The coefficients in front of each formula tell you how many atoms of each reactant are required for the reaction to go to completion
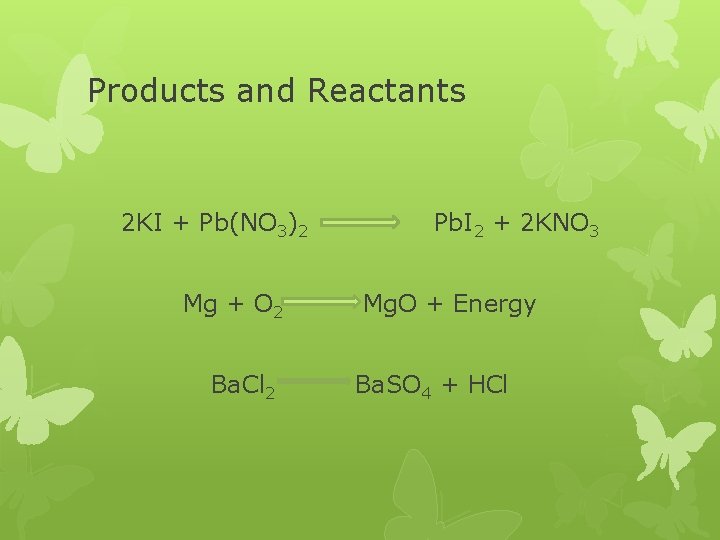
Products and Reactants 2 KI + Pb(NO 3)2 Mg + O 2 Ba. Cl 2 Pb. I 2 + 2 KNO 3 Mg. O + Energy Ba. SO 4 + HCl

Chemical Equations 2 The substances on the left side undergo a reaction: they are the reactants The substances on the right side of the equation are the products of the reaction This type of chemical reaction can be reduced to a net ionic equation by removing all the components that do not actually react but rather stay in solution

Balancing Chemical Equations There a few simple steps that will enable you to balance any chemical equation 1. Count the atoms of each element in the equation in its unbalanced form. Write the number of each element above that element. 2. Balance the atom or polyatomic ion that appears only once on each side by inserting a coefficient in front of the whole formula.

Balancing Chemical Equations 2 3. Balance the other elements and polyatomic ions one by one, leaving O and H for last. 4. Check your work by counting the atoms of each element on both sides of the arrow. Note: you cannot change the subscripts in the formulas since this would change the substance in the reaction!

Balancing Chemical Equations Some other things to keep in mind: Treat polyatomic ions as units if they appear on both sides of an equation, as they typically will. Do not count the atoms inside the polyatomic ion since you might get them confused with the same atoms that are not part of the ion. Sometimes a change you make in the middle of the balancing process will cause one of the other elements or polyatomic ions to go out of balance. Don’t let this bother you: you will just go back and update the coefficients as you go along.
Balancing Equations Think in terms of the mathematical concept of least common multiples (LCMs). You can figure out how many atoms or ions are on each side of the equation; just find the LCM of the two numbers to balance that unit. The concept of the LCM is important because the ratios between substances in the reaction should be expressed in lowest terms
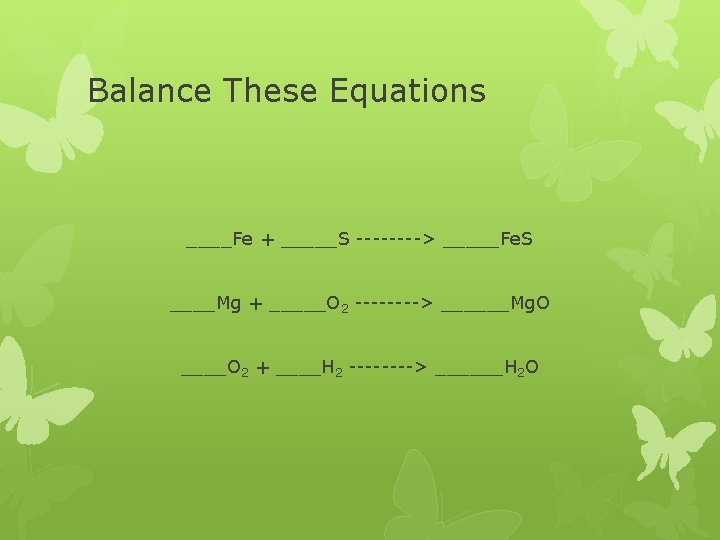
Balance These Equations ____Fe + _____S ----> _____Fe. S ____Mg + _____O 2 ----> ______Mg. O ____O 2 + ____H 2 ----> ______H 2 O
- Slides: 20