CHEMICAL REACTIONS AND EQUATIONS Chapter 9 Chemical Reaction



























- Slides: 49

CHEMICAL REACTIONS AND EQUATIONS Chapter 9

Chemical Reaction • A process in which one or more substances are converted into new substances with different chemical and physical properties. • Not reversible.

Chemical Reactions • Examples: • Breathing • Burning gasoline • Baking • All chemical reactions involve two types of substances: • Reactant – a substance that enters into a chemical reaction • Product – a substance that is produced by a chemical reaction

Reasons for Reactions • The arrangement of electrons in an atom determines whether it will bond with other atoms and with which atoms it will bond. • An atom with a full set of valence electrons will not form bonds. • An atom with an incomplete set of valence electrons will bond.

HOMEWORK Pg. 281 1 -4

CHEMICAL EQUATIONS 9 -2

Chemical Equations • Chemical reactions are represented by sentences known as chemical equations. • A chemical equation identifies the reactants and products in a chemical reaction.

Chemical Equations • A chemical reaction is the process by which one or more substances are changed into one or more different substances • It can be represented by an equation. • It shows what changes have taken place • It also shows the relative amounts of the various elements and compounds that take part in these reactions

Chemical Equations • The starting substances in a chemical reaction are the reactants • The substances that are formed by the chemical reaction are the products • Reactant(g) + Reactants(g) Products(g) + Products(g)

Chemical Equations • The letters in parentheses indicate the physical state of each substance involved. • (g) means gas • (l) means liquid • (s) means solid • (aq) means that it is dissolved in water

Word Equations • Gives names of the reactants and names of products. • Example: • calcium + oxygen calcium oxide • + means “reacts with” • means “yields”, indicates direction of reaction.

Formula Equations • Writing a chemical equation: • First determine the symbols and formulas that describe the reactants and products. • Substitute into the word equation. • Example: calcium + oxygen calcium oxide • Ca + O 2 Ca. O

Practice Problems • Silver (I) nitrate reacts with copper to form copper (II) nitrate and silver. • Hydrogen peroxide (H 2 O 2) decomposes to form water and oxygen gas.

Balancing Chemical Equations • Law of conservation of mass: • Matter is neither created nor destroyed. • Total mass of the reactants must be equal to the total mass of the products. • For mass to remain constant before and after a chemical reaction, the number of atoms of each element must be the same before and after a chemical reaction.

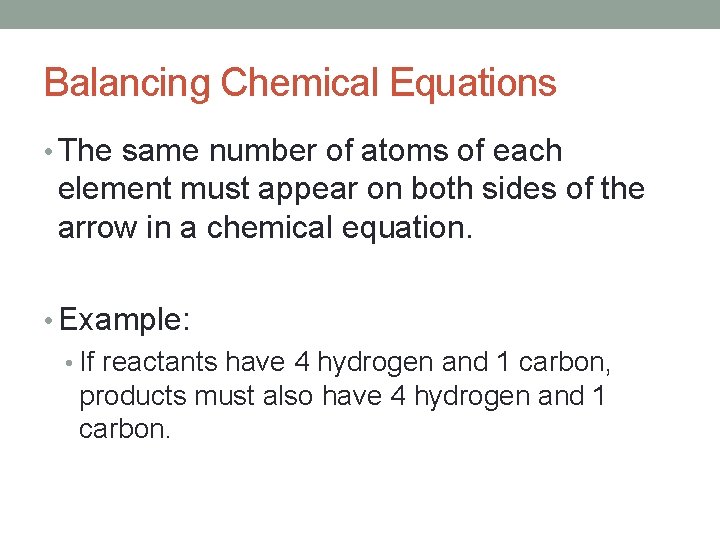
Balancing Chemical Equations • The same number of atoms of each element must appear on both sides of the arrow in a chemical equation. • Example: • If reactants have 4 hydrogen and 1 carbon, products must also have 4 hydrogen and 1 carbon.

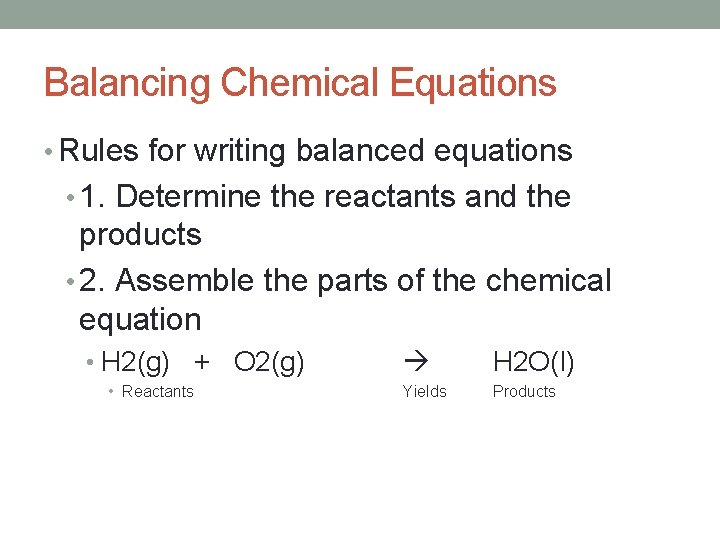
Balancing Chemical Equations • Rules for writing balanced equations • 1. Determine the reactants and the products • 2. Assemble the parts of the chemical equation • H 2(g) + O 2(g) • Reactants H 2 O(l) Yields Products

Balancing Chemical Equations • 3. Write a balanced equation • Balancing means showing an equal number of atoms for each element on both sides of the equation • Remember nothing is lost or gained! • Only Transferred • When balancing equations only change the coefficients • Never change the subscripts!! • Finally make sure that all the coefficients are in the lowest possible ratio.

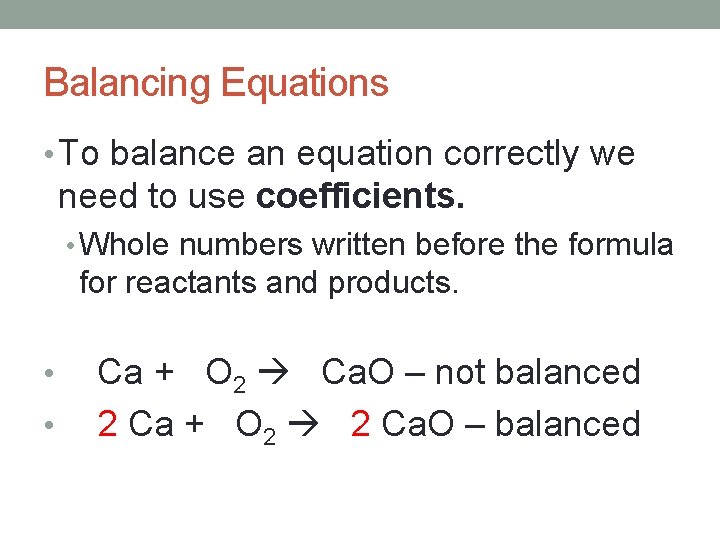
Balancing Equations • Ca + O 2 Ca. O • Not balanced. • 2 oxygen on the reactants, 1 oxygen in the products. • *Equation cannot be balanced by changing subscripts* • Changing a subscript changes the identity of the substance.

Balancing Equations • To balance an equation correctly we need to use coefficients. • Whole numbers written before the formula for reactants and products. • • Ca + O 2 Ca. O – not balanced 2 Ca + O 2 2 Ca. O – balanced

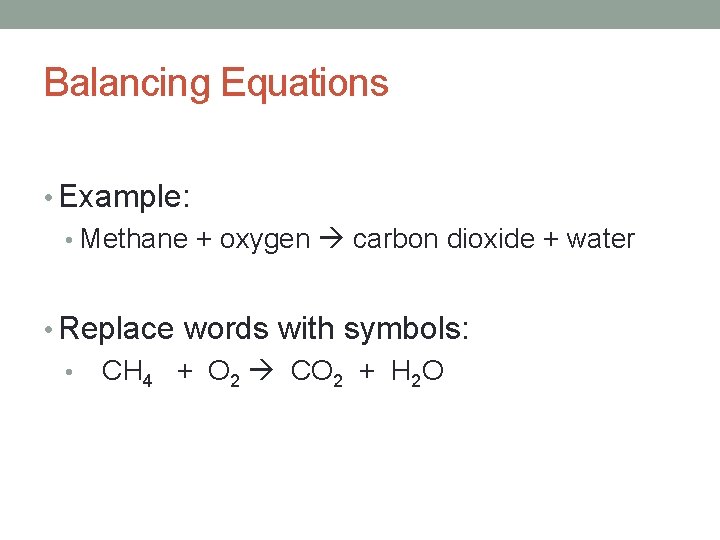
Balancing Equations • Example: • Methane + oxygen carbon dioxide + water • Replace words with symbols: • CH 4 + O 2 CO 2 + H 2 O

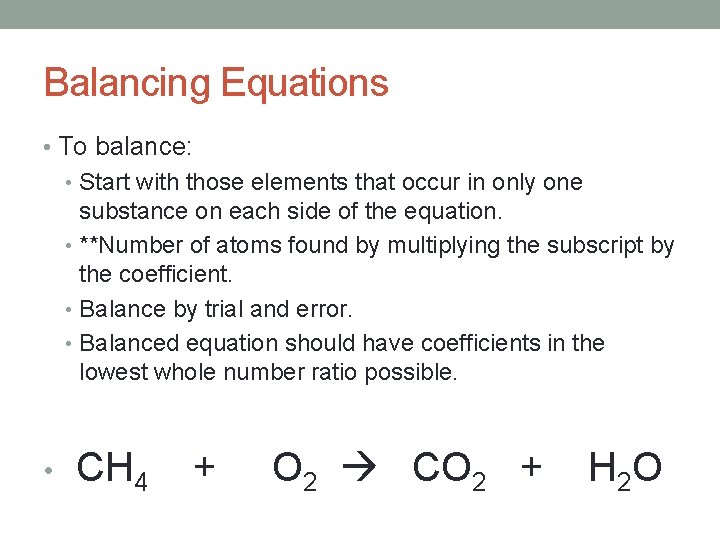
Balancing Equations • To balance: • Start with those elements that occur in only one substance on each side of the equation. • **Number of atoms found by multiplying the subscript by the coefficient. • Balance by trial and error. • Balanced equation should have coefficients in the lowest whole number ratio possible. • CH 4 + O 2 CO 2 + H 2 O

Practice Problems • Balance the following equation: • H 2(g) + O 2(g) H 2 O(l)

Practice Problems • 2 H 2(g) + O 2(g) 2 H 2 O(l) H=4 O=2 • The equation is balanced

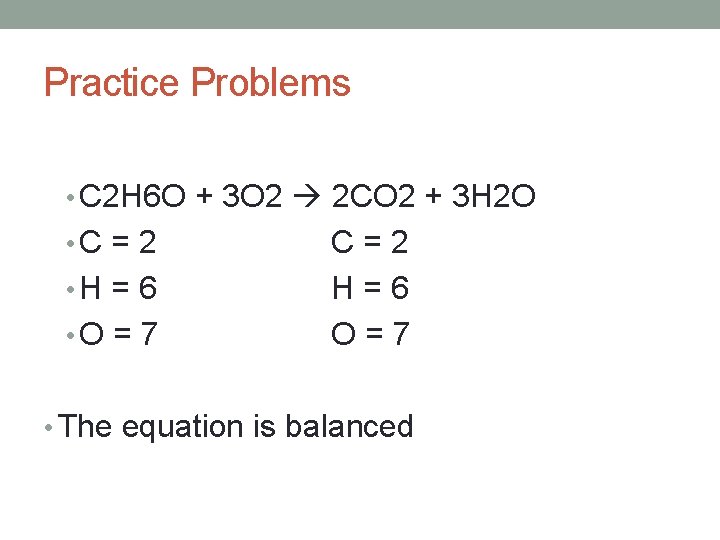
Practice Problems • Balance the following equation: • C 2 H 6 O + O 2 CO 2 + H 2 O

Practice Problems • C 2 H 6 O + 3 O 2 2 CO 2 + 3 H 2 O • C = 2 • H = 6 • O = 7 C=2 H=6 O=7 • The equation is balanced

Practice Problems • Balance the following equations: • 1. Zn + HCl ----> Zn. Cl 2 + H 2 • 2. Al + O 2 -----> Al 2 O 3 • 3. Al + Cu. SO 4 ------> Al 2(SO 4)3 + Cu • 4. Li + H 2 O ------> Li. OH + H 2

Practice Problems • Aluminum reacts with oxygen to produce aluminum oxide. • Sodium nitrate reacting with calcium chloride to produce sodium chloride and calcium nitrate • Dinitrogen pentoxide reacts with water to produce nitric acid (HNO 3)

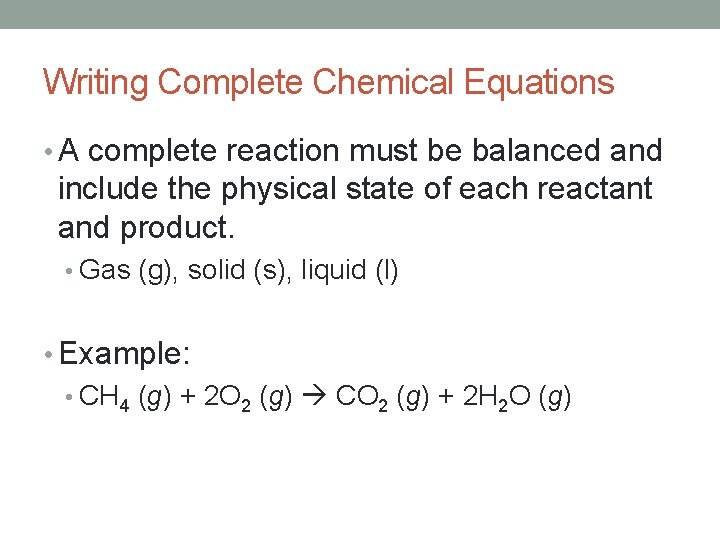
Writing Complete Chemical Equations • A complete reaction must be balanced and include the physical state of each reactant and product. • Gas (g), solid (s), liquid (l) • Example: • CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g)

HOMEWORK Pg. 290 1 -4

CLASSIFYING CHEMICAL REACTIONS 9 -3

Type of Reactions • Four types of chemical reactions. • Direct combination (Synthesis) • Decomposition • Single-replacement • Double-replacement

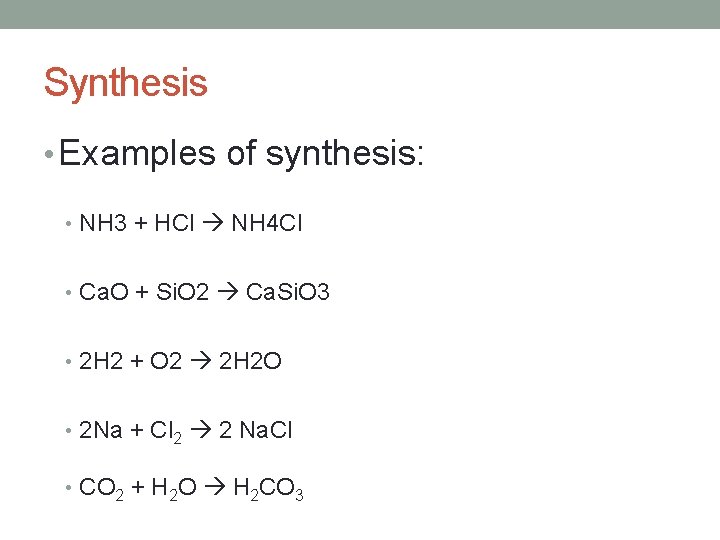
Synthesis • Two or more reactants come together to form a single product. • Synthesis reactions. • A + B AB • Product is always more complex than either of the reactants.

Synthesis • Synthesis – two or more substances combine to form one new substance • It follows this general form: • Element or compound + Element or compound Compound

Synthesis • Examples of synthesis: • NH 3 + HCl NH 4 Cl • Ca. O + Si. O 2 Ca. Si. O 3 • 2 H 2 + O 2 2 H 2 O • 2 Na + Cl 2 2 Na. Cl • CO 2 + H 2 O H 2 CO 3

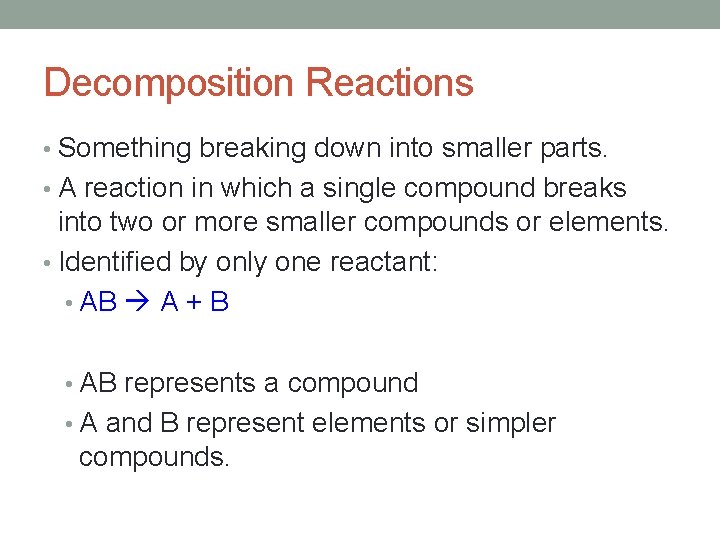
Decomposition Reactions • Something breaking down into smaller parts. • A reaction in which a single compound breaks into two or more smaller compounds or elements. • Identified by only one reactant: • AB A + B • AB represents a compound • A and B represent elements or simpler compounds.

Decomposition Reactions • Decomposition – when a compound is breaking apart or decompose into simpler substances when energy is supplied • Energy may be supplied in the form of heat, light, mechanical shock, or electricity • It follows this general form: • Compound Two or more elements or compounds

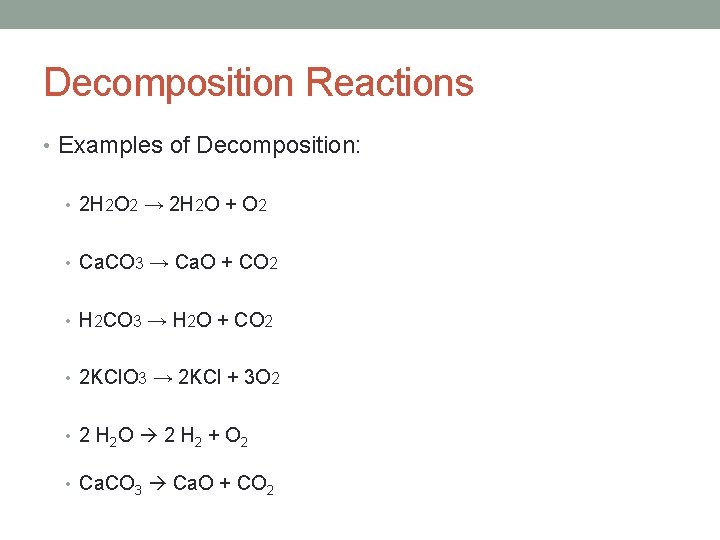
Decomposition Reactions • Examples of Decomposition: • 2 H 2 O 2 → 2 H 2 O + O 2 • Ca. CO 3 → Ca. O + CO 2 • H 2 CO 3 → H 2 O + CO 2 • 2 KCl. O 3 → 2 KCl + 3 O 2 • 2 H 2 O 2 H 2 + O 2 • Ca. CO 3 Ca. O + CO 2

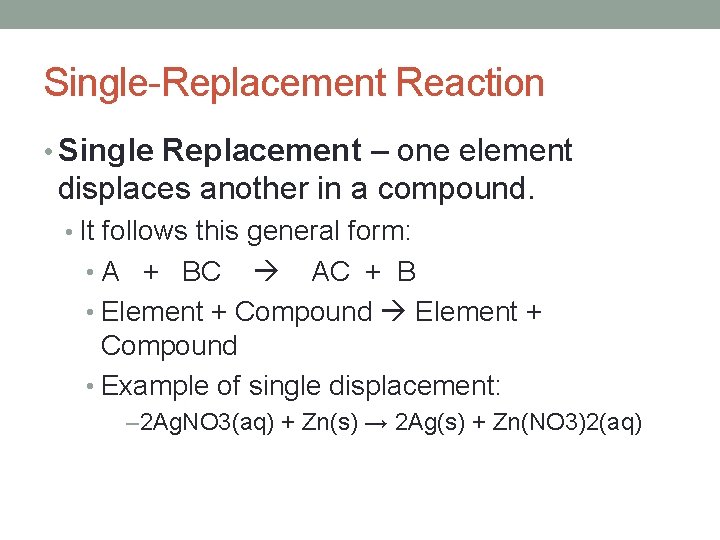
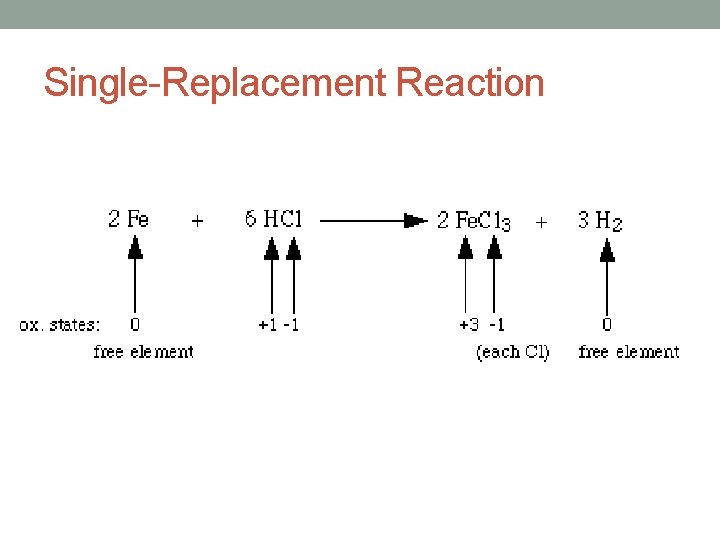
Single-Replacement Reaction • Single Replacement – one element displaces another in a compound. • It follows this general form: • A + BC AC + B • Element + Compound • Example of single displacement: – 2 Ag. NO 3(aq) + Zn(s) → 2 Ag(s) + Zn(NO 3)2(aq)

Single-Replacement Reaction

Single-Replacement Reaction • An uncombined element displaces an element that is part of a compound. • Reactants: one element and one compound • A + BX AX + B • Generally ionic compounds.

Single-Replacement Reaction • Example: • Mg + Cu. SO 4 Mg. SO 4 +Cu • A more active element will replace a less active element. • Figure 9 -19 – activity series: • Used to predict whether or not a single-replacement reaction will occur. • An element can replace any element that is below it. • Metals replace metals or hydrogen • Nonmetals replace nonmetals • Example: • Cl 2 + 2 KI ? • Cl 2 + 2 KI KCl + I 2

Double-Replacement Reaction • Double Replacement – is similar to single displacement but it uses two compounds instead of one element • It follows this general form: • Compound + Compound • AB + CD → AD + CB

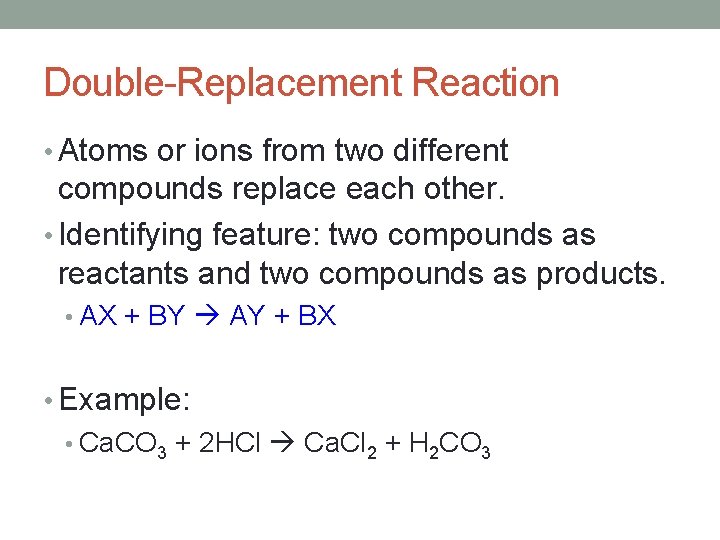
Double-Replacement Reaction • Atoms or ions from two different compounds replace each other. • Identifying feature: two compounds as reactants and two compounds as products. • AX + BY AY + BX • Example: • Ca. CO 3 + 2 HCl Ca. Cl 2 + H 2 CO 3

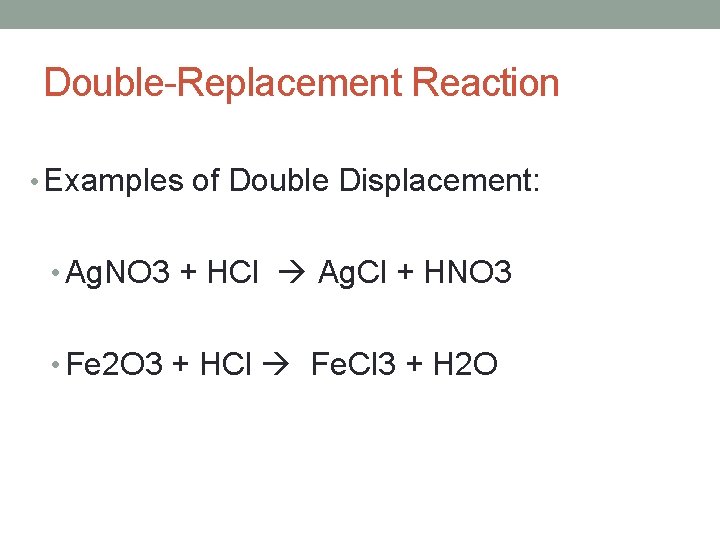
Double-Replacement Reaction • Examples of Double Displacement: • Ag. NO 3 + HCl Ag. Cl + HNO 3 • Fe 2 O 3 + HCl Fe. Cl 3 + H 2 O

Double-Replacement Reaction • Conditions: • Most reactions will not occur unless the reactants are dissolved in water so that the ions can separate into ions. • Reactions are more likely to take place if one of the products is a molecular compound, a precipitate, or a gas.

Combustion • Combustion – occurs when a compound burns in air, it is actually reacting with the oxygen in the air. • The products of the oxidation of the hydrocarbon under normal conditions are carbon dioxide and water vapor. • Combustion reactions are refered to as oxidation reactions. • They follow this general form: • Hydrocarbon + Oxygen Carbon Dioxide + Water (MOST USED) • Hydrocarbon + Flourine Carbon Flouride + Hydrogen Flouride

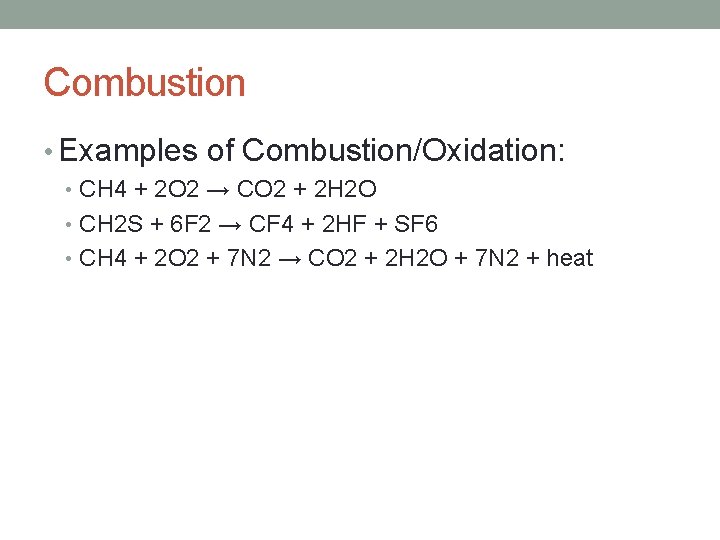
Combustion • Examples of Combustion/Oxidation: • CH 4 + 2 O 2 → CO 2 + 2 H 2 O • CH 2 S + 6 F 2 → CF 4 + 2 HF + SF 6 • CH 4 + 2 O 2 + 7 N 2 → CO 2 + 2 H 2 O + 7 N 2 + heat

Combustion • Not all reactions take one of these five general forms. • There are other classes of reactions, but those we will talk about in a later chapter.

HOMEWORK Pg. 297 1 -4