Chemical Reactions and Enzymes Energy and Matter n
















- Slides: 24

Chemical Reactions and Enzymes

Energy and Matter n Energy n The ability to do work or cause change n Occurs in various forms n Can be converted to another form n Forms important to biological systems are chemical, thermal, electrical and mechanical energy n Free energy is the energy in a system that is available for work

States of Matter n n Atoms are in constant motion The rate at which atoms or molecules in a substance move determines its state

States of Matter n Solid n Molecules are tightly linked. n Little energy n Liquid n Molecules are not as tightly linked n Medium amount of energy

States of Matter n. Gas n. Molecules have little or no attraction to each other n. Fill the volume of the occupied container n. Move most rapidly n To cause a substance to change state, thermal energy (heat) must be added to or removed from a substance

Energy and Chemical Reactions n Living things undergo thousands of chemical reactions as part of the life process

Energy and Chemical Reactions n n Many are very complex involving multistep sequences called biochemical pathways Chemical equations represent chemical reactions n Reactants are shown on the left side of the equation n Products are shown on the right side n A+B C+D

Energy Transfer n Much of the energy organisms need is provided by sugar (food) n n Undergoes a series of chemical reactions in which energy is released (cell respiration) The net release of free energy is called an exothermic reaction

Energy Transfer n n Reactions that involve a net absorption of free energy are called endothermic reactions Photosynthesis is an example Mix Barium hydroxide and aluminum salt, and the products dissolve in water of hydration. This is VERY COLD!

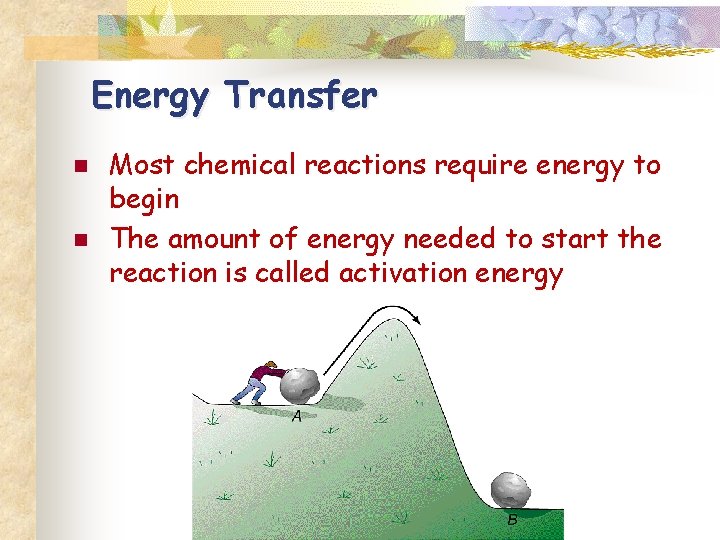
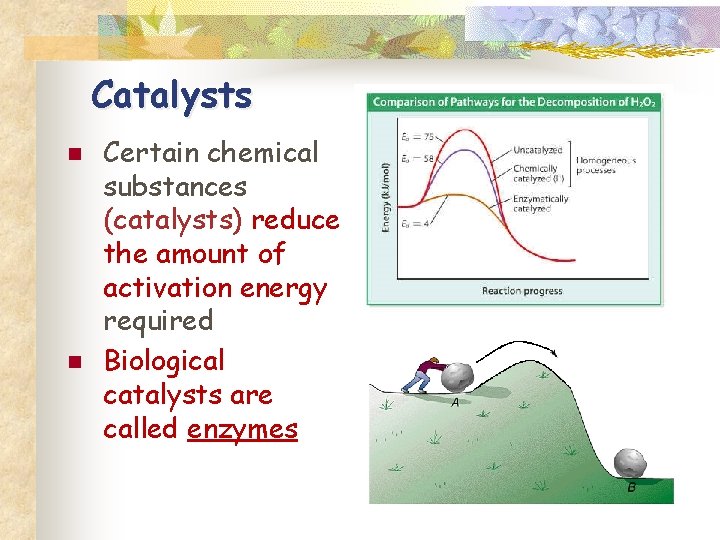
Energy Transfer n n Most chemical reactions require energy to begin The amount of energy needed to start the reaction is called activation energy

Catalysts n n Certain chemical substances (catalysts) reduce the amount of activation energy required Biological catalysts are called enzymes

Catalysts n Enzymes are an important class of catalysts in living organisms n Mostly protein n Thousands of different kinds n Each specific for a different chemical reaction

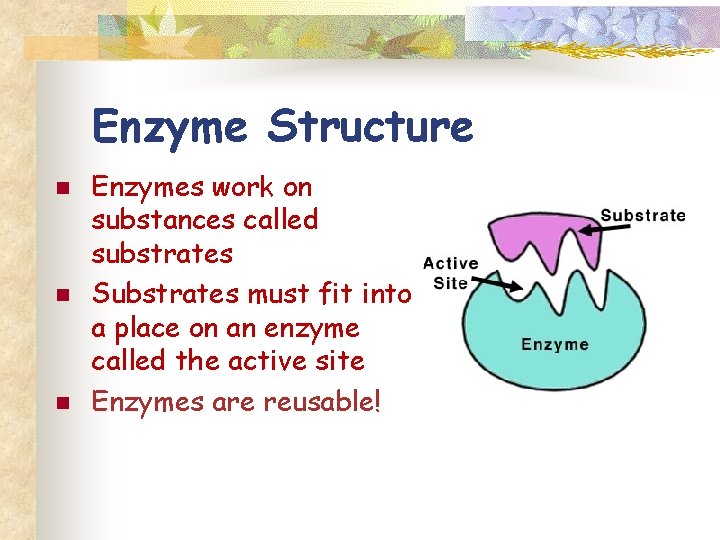
Enzyme Structure n n n Enzymes work on substances called substrates Substrates must fit into a place on an enzyme called the active site Enzymes are reusable!

Solutions

Solutions n A solution is a mixture in which 2 or more substances are uniformly distributed in another substance

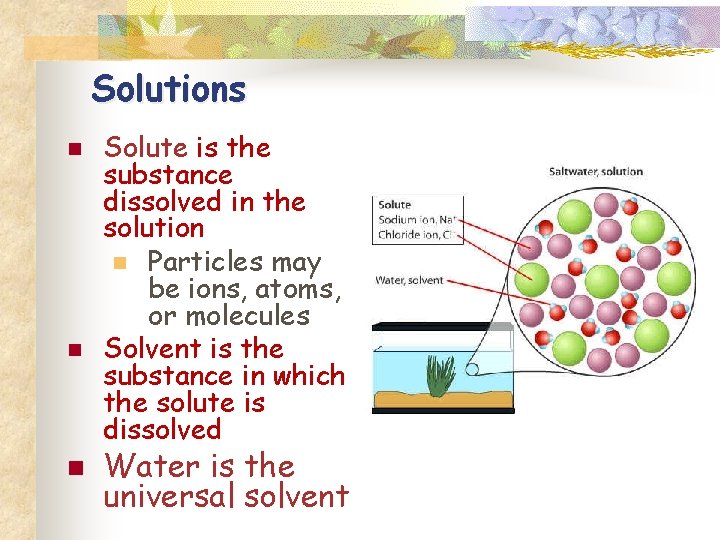
Solutions n n n Solute is the substance dissolved in the solution n Particles may be ions, atoms, or molecules Solvent is the substance in which the solute is dissolved Water is the universal solvent

Acids and Bases n One of the most important aspects of a living system is the degree of acidity or alkalinity

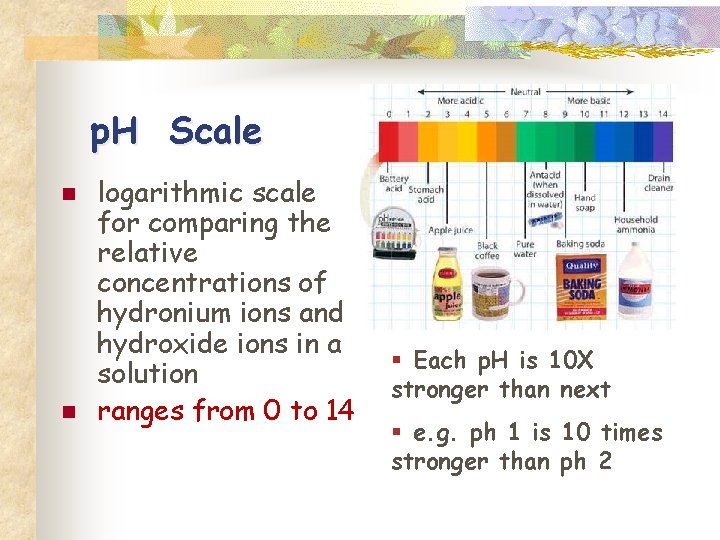
p. H Scale n n logarithmic scale for comparing the relative concentrations of hydronium ions and hydroxide ions in a solution ranges from 0 to 14 § Each p. H is 10 X stronger than next § e. g. ph 1 is 10 times stronger than ph 2

Acids n n n Compounds that donate a proton (H+) when dissolved in a solution. the lower the p. H the stronger the acid 0 -6 on the p. H scale n HCl H+ + Cl-

Bases n n n Compounds that accepts a proton (H+) when dissolved in a solution. the higher the p. H the stronger the base 8 -14 on the p. H scale n Na. OH Na+ + OH-

Acids and Bases n p. H 7. 0 is neutral

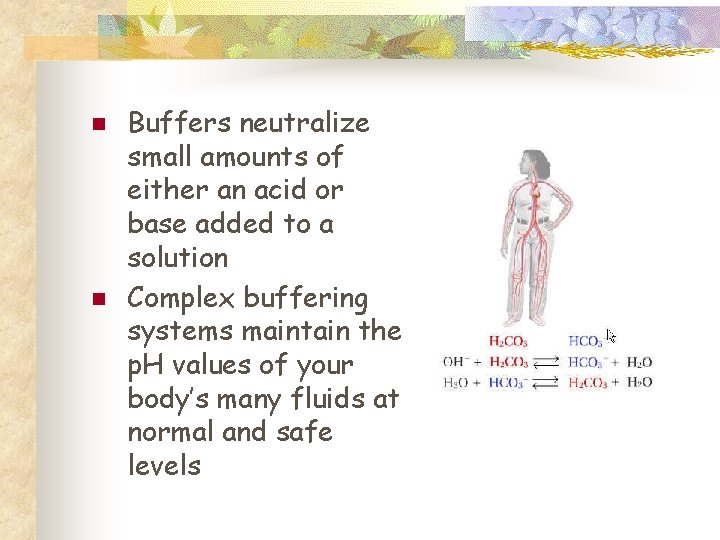
Buffers n n Control of p. H is very important Most enzymes function only within a very narrow p. H Control is accomplished with buffers made by the body Buffers keep a neutral p. H (p. H 7)

n n Buffers neutralize small amounts of either an acid or base added to a solution Complex buffering systems maintain the p. H values of your body’s many fluids at normal and safe levels

 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Biology-roots.com
Biology-roots.com Enzymes affect the reaction in living cells by changing the
Enzymes affect the reaction in living cells by changing the Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Apoenzyme
Apoenzyme Ecological succession
Ecological succession How to write half reactions
How to write half reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemical nature of enzymes
Chemical nature of enzymes Reactants and products
Reactants and products Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review I intro
I intro Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chemical reactions of copper and percent yield
Chemical reactions of copper and percent yield Chemical bond vocabulary
Chemical bond vocabulary