Chemical Reactions and Enzymes Chemical Symbols Element proton

Chemical Reactions and Enzymes
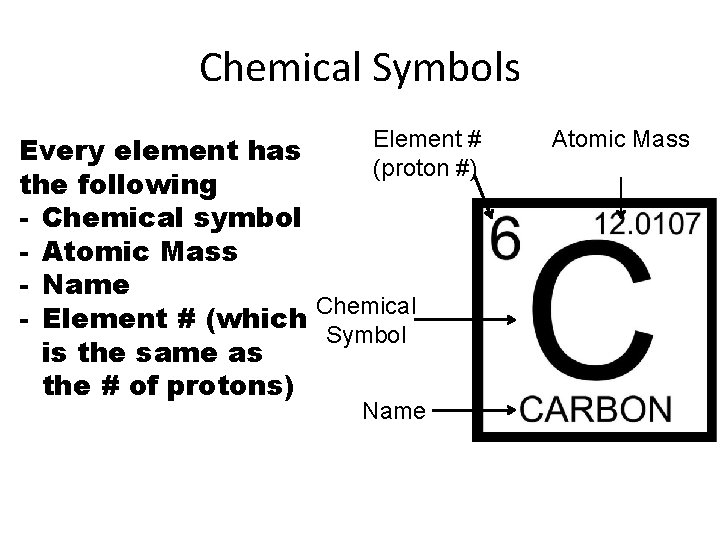
Chemical Symbols Element # (proton #) Every element has the following - Chemical symbol - Atomic Mass - Name Chemical - Element # (which Symbol is the same as the # of protons) Name Atomic Mass

Introduction to Chemical Reactions Making new substances

Main Ideas Chemical Reactions are represented by Chemical Equations are balanced to show the same number of atoms of each element on each side. The Law of Conservation of Mass says that atoms won’t be created or destroyed in a chemical reaction. That is why you have to balance chemical equations!
Chemical Reactions are Everywhere Cooking Respiration

Chemical Reactions are Everywhere Hair Dye Auto Fuel

How do you know when a chemical reaction takes place? Color Change Precipitate Formation

How do you know when a chemical reaction takes place? Gas Formation Odor
How do you know when a chemical reaction takes place? Temperature Change in Acidity
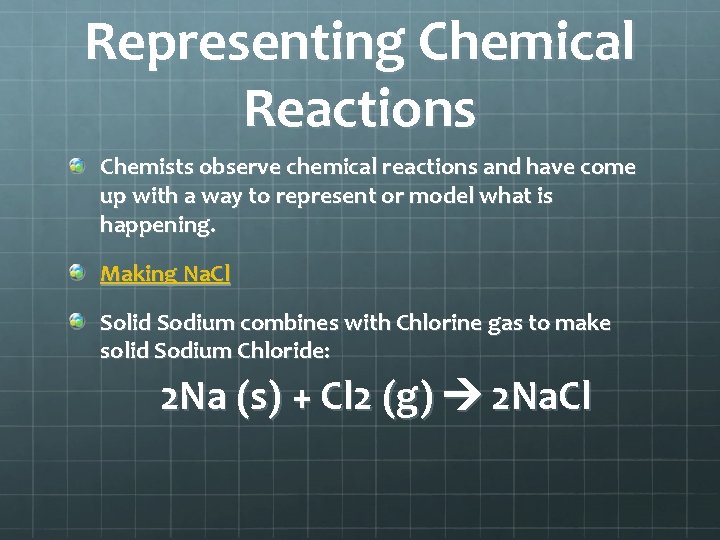
Representing Chemical Reactions Chemists observe chemical reactions and have come up with a way to represent or model what is happening. Making Na. Cl Solid Sodium combines with Chlorine gas to make solid Sodium Chloride: 2 Na (s) + Cl 2 (g) 2 Na. Cl
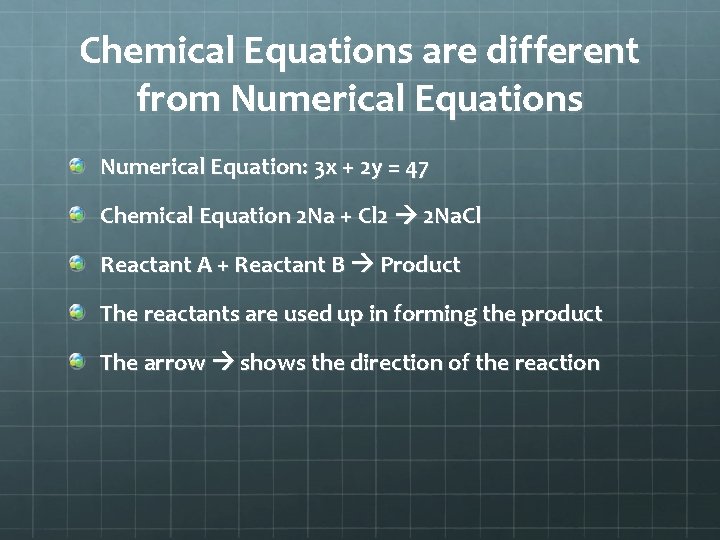
Chemical Equations are different from Numerical Equations Numerical Equation: 3 x + 2 y = 47 Chemical Equation 2 Na + Cl 2 2 Na. Cl Reactant A + Reactant B Product The reactants are used up in forming the product The arrow shows the direction of the reaction
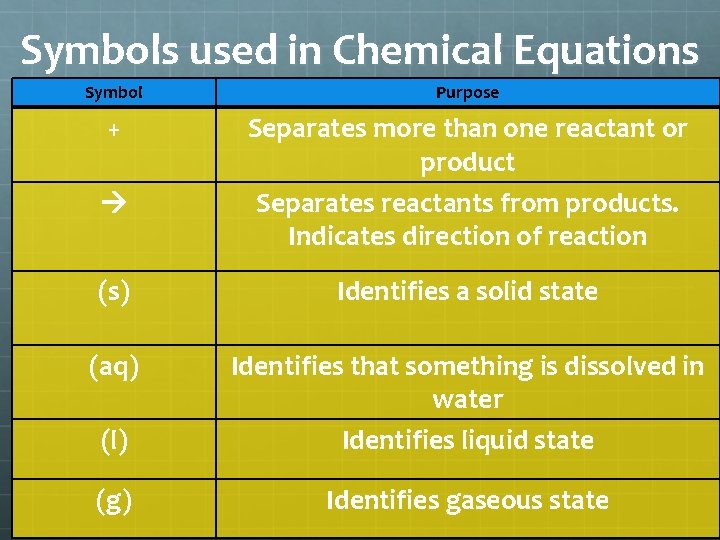
Symbols used in Chemical Equations Symbol Purpose + Separates more than one reactant or product Separates reactants from products. Indicates direction of reaction (s) Identifies a solid state (aq) (l) Identifies that something is dissolved in water Identifies liquid state (g) Identifies gaseous state

Law of Conservation of Mass In a chemical reaction, matter is neither created nor destroyed. Atoms won’t change their identity (e. g. a Carbon atom can’t become an Iron atom) This means that you have to have the same number of each type of atom on each side of the chemical equation. Conservation of Mass Video

Balancing Equations After you write a chemical equation you have to balance it to make sure that the same number of atoms of each element are on each side. How would you balance this equation? Li + H 2 O H 2 + Li. OH
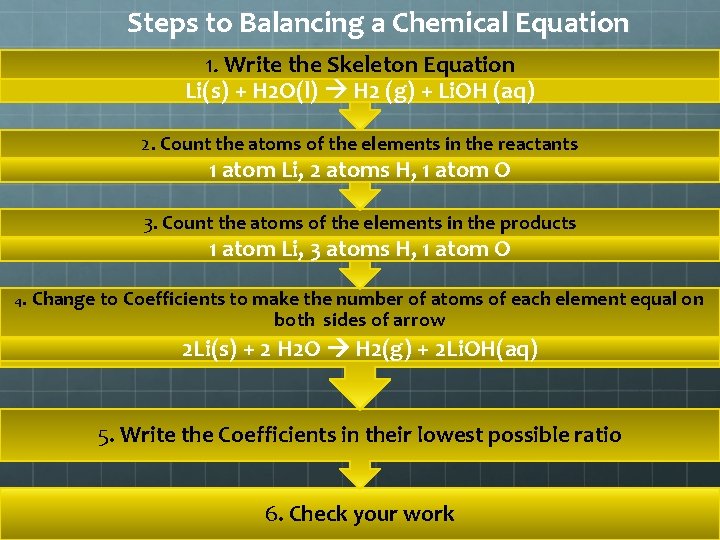
Steps to Balancing a Chemical Equation 1. Write the Skeleton Equation Li(s) + H 2 O(l) H 2 (g) + Li. OH (aq) 2. Count the atoms of the elements in the reactants 1 atom Li, 2 atoms H, 1 atom O 3. Count the atoms of the elements in the products 1 atom Li, 3 atoms H, 1 atom O 4. Change to Coefficients to make the number of atoms of each element equal on both sides of arrow 2 Li(s) + 2 H 2 O H 2(g) + 2 Li. OH(aq) 5. Write the Coefficients in their lowest possible ratio 6. Check your work

Another Example CH 4 (methane gas) + O 2 CO 2 + H 2 O Reactants Products # of Carbons = 1 # of Hydrogens = 4 # of Hydrogens = 2 # of Oxygens = 3 Total atoms = 7 Total atoms = 6 7 ≠ 6! Where did our atoms go?
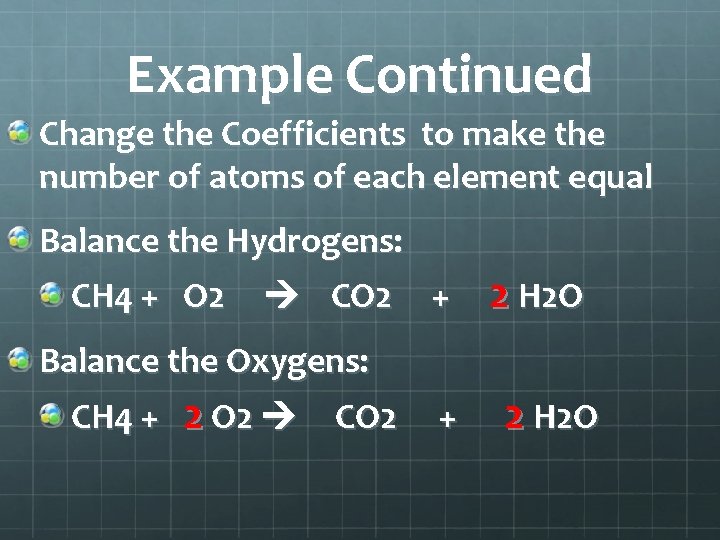
Example Continued Change the Coefficients to make the number of atoms of each element equal Balance the Hydrogens: CH 4 + O 2 CO 2 + 2 H 2 O Balance the Oxygens: CH 4 + 2 O 2 CO 2 + 2 H 2 O
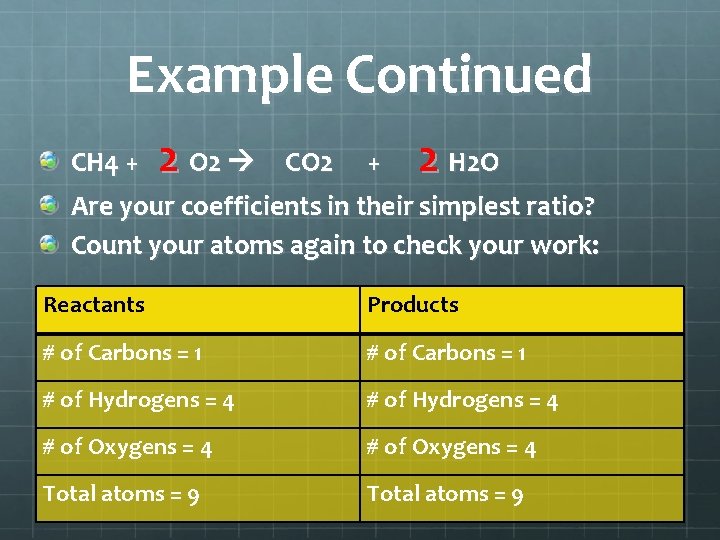
Example Continued CH 4 + 2 O 2 CO 2 + 2 H 2 O Are your coefficients in their simplest ratio? Count your atoms again to check your work: Reactants Products # of Carbons = 1 # of Hydrogens = 4 # of Oxygens = 4 Total atoms = 9

Try These! H 2 O -> H 2 + O 2 C 6 H 12 O 6 + O 2 -> CO 2 + H 2 O Think – Pair - Share
Review Matter is not destroyed or created Atoms are rearranged in chemical reactions Chemical equations represent chemical reactions You have to have the same number of each type of atom on the left and right hand side of a chemical equation
Chemistry and Life One unromantic yet productive way of viewing life is to see it as a set of coordinated chemical reactions. This leads to an obvious question – What determines what chemical reactions are possible?
Chemical Reactions Whether a chemical reaction will or won’t occur under particular conditions is determined by the laws of thermodynamics. Keeping it simple If the overall amount of order is decreased by a reaction, the reaction is wanted an so requires little energy to get started. <SPONTANEOUS> - Products @ a lower energy level than the reactants If the overall amount of order is increased by a reaction, the reaction is not wanted and so requires lots of energy to get started. <NON-SPONTANEOUS) Products @ a higher energy level than the reactants

The Direction of Spontaneous Reactions (and what it takes to go the other way) “Non-Spontaneous” Reaction – Takes energy to go this way
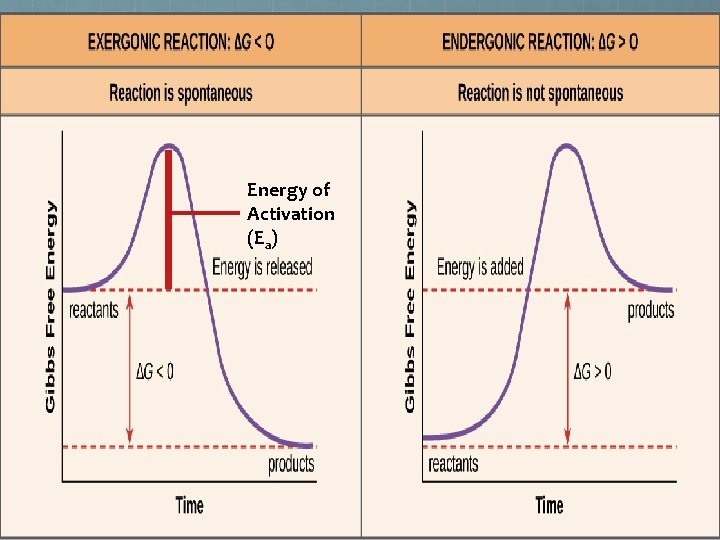
Energy of Activation (Ea)

ENZYMES Proteins that make YOU possible! - Speed up RXNs that happen too slowly to support life - Speed up RXNs necessary to survive/maintain homeostasis - Involved in the construction and maintenance of your body - Reduces the amount of energy needed to start a RXN Cool Features of Enzymes - Made of proteins - Like any tool, can be reused thousands of times before needing to be replaced (enzymes are easily recycled by your body to make new enzymes) - Can be turned on or turned off depending on if they are needed - Most work for free -> no energy investment needed… though providing some energy can supercharge the enzyme to work even faster. Copyright © 2009 Pearson Education, Inc.
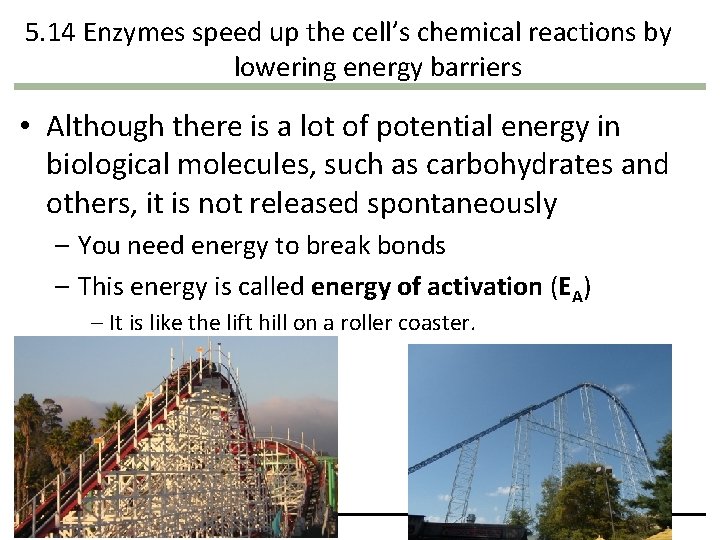
5. 14 Enzymes speed up the cell’s chemical reactions by lowering energy barriers • Although there is a lot of potential energy in biological molecules, such as carbohydrates and others, it is not released spontaneously – You need energy to break bonds – This energy is called energy of activation (EA) – It is like the lift hill on a roller coaster. Copyright © 2009 Pearson Education, Inc.
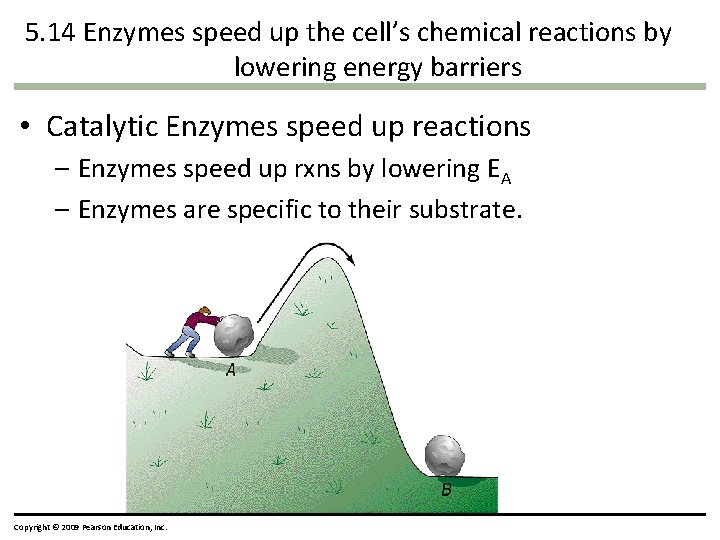
5. 14 Enzymes speed up the cell’s chemical reactions by lowering energy barriers • Catalytic Enzymes speed up reactions – Enzymes speed up rxns by lowering EA – Enzymes are specific to their substrate. Copyright © 2009 Pearson Education, Inc.
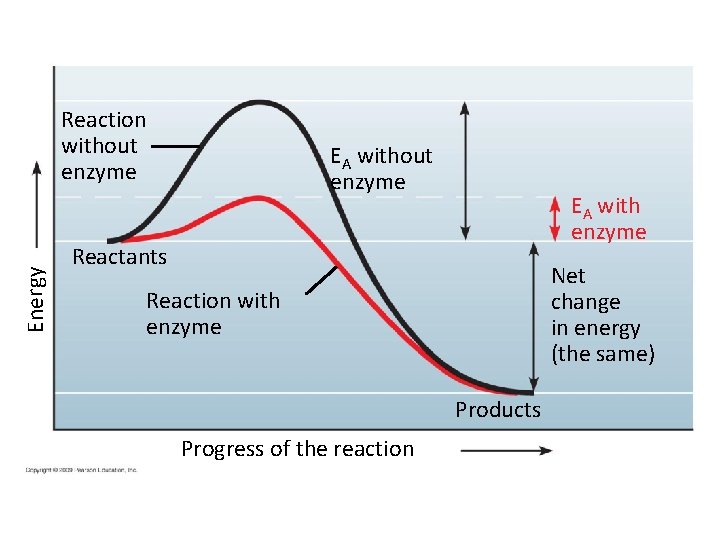
Energy Reaction without enzyme EA with enzyme Reactants Net change in energy (the same) Reaction with enzyme Products Progress of the reaction
5. 15 A specific enzyme catalyzes each cellular reaction • Enzymes have unique three-dimensional shapes – The shape is critical to their role as biological catalysts – The shape includes an active site – spot where substrate binds – Enzymes will convert substrate to products Youase Copyright © 2009 Pearson Education, Inc.
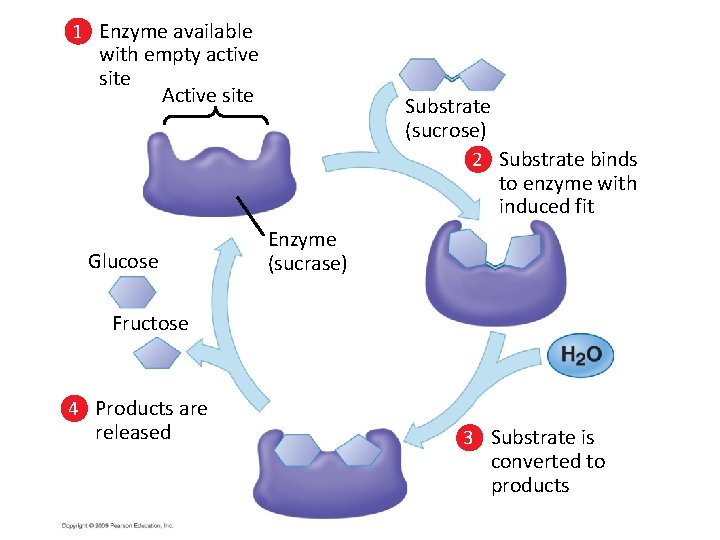
1 Enzyme available with empty active site Active site Glucose Substrate (sucrose) 2 Substrate binds to enzyme with induced fit Enzyme (sucrase) Fructose 4 Products are released 3 Substrate is converted to products
5. 15 A specific enzyme catalyzes each cellular reaction • For optimum activity, enzymes require certain “Just right” environmental conditions – Temperature and p. H Copyright © 2009 Pearson Education, Inc.
Controlling Enzymes • Inhibitors are chemicals that inhibit an enzyme’s activity – A substance that competes for the same active site as the substrate is called a competitive inhibitor. Example of a common competitive inhibitor for your attention. Copyright © 2009 Pearson Education, Inc.
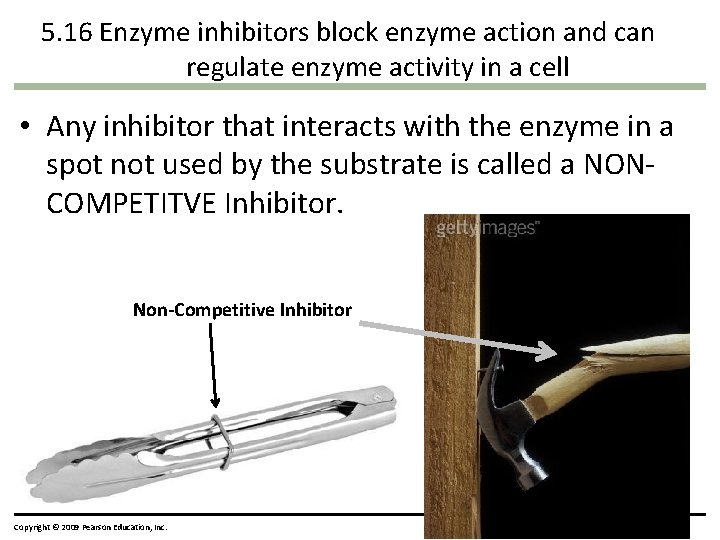
5. 16 Enzyme inhibitors block enzyme action and can regulate enzyme activity in a cell • Any inhibitor that interacts with the enzyme in a spot not used by the substrate is called a NONCOMPETITVE Inhibitor. Non-Competitive Inhibitor Copyright © 2009 Pearson Education, Inc.
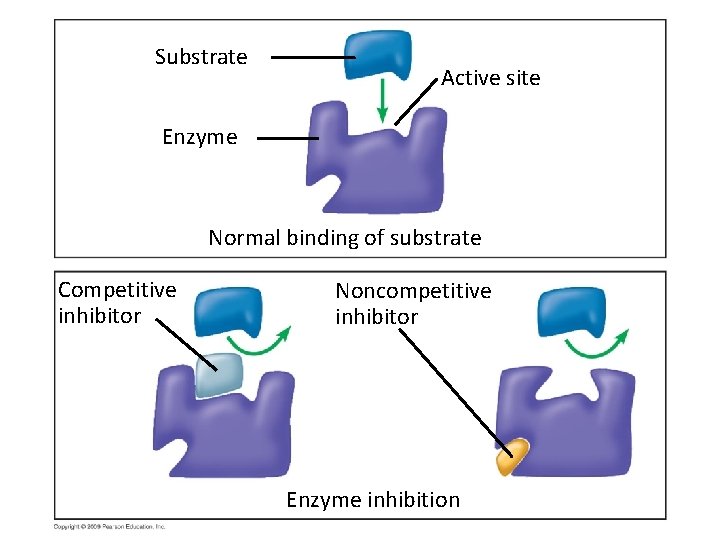
Substrate Active site Enzyme Normal binding of substrate Competitive inhibitor Noncompetitive inhibitor Enzyme inhibition
- Slides: 34