CHEMICAL REACTIONS AND COMBUSTION MRS BROSTROM INTEGRATED SCIENCE
CHEMICAL REACTIONS AND COMBUSTION MRS. BROSTROM INTEGRATED SCIENCE C
OBJECTIVE: • Describe combustion reactions of hydrocarbons and their resulting by-products.

FORMING NEW SUBSTANCES • A chemical reaction is a process in which one or more substances to make one or more new substances • Chemical and physical properties differ from the original substance • For example: mixing baking powder and water form CO 2 and give muffins there sponge-like texture

HOW CAN YOU TELL THAT A CHEMICAL REACTION IS TAKING PLACE? • Gas formation • Solid formation- a precipitate is a solid substance that is formed in a solution • Energy exchange- energy is released as light or heat or energy can be absorbed • Color change Oxidation of metal (Seawater reacting with metal, forms a solid.

SUGAR SNAKE • However, these signs do not guarantee a chemical reaction is taking place • The most important sign is the formation of new substances that have different properties • For example, combining sugar and sulfuric acid; bubbles form, a gas is given off, and the beaker becomes hot • But most important, a new substance is formed with different properties
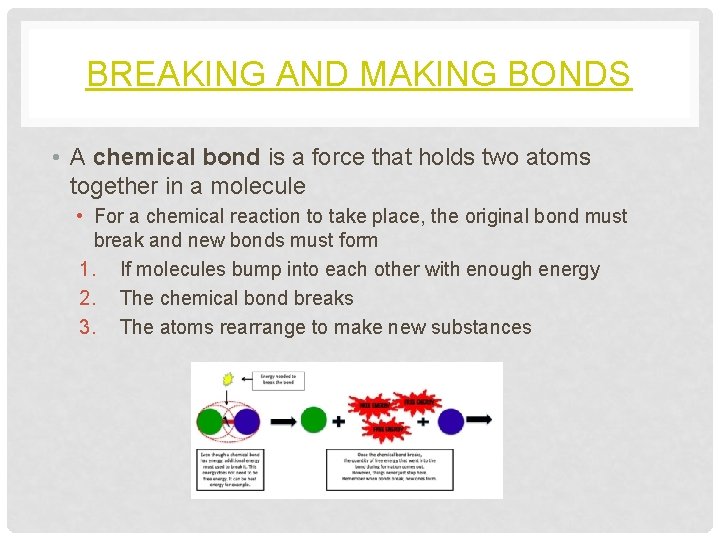
BREAKING AND MAKING BONDS • A chemical bond is a force that holds two atoms together in a molecule • For a chemical reaction to take place, the original bond must break and new bonds must form 1. If molecules bump into each other with enough energy 2. The chemical bond breaks 3. The atoms rearrange to make new substances

NEW BONDS MAKE NEW SUBSTANCES • Sodium is a metal that reacts violently in water • Chlorine gas is poisonous • But when Na and Cl react, they result in a new substance, Na. Cl or salt • Salt is a harmless substance with very different properties +
COMBUSTION – type of chemical reaction – occurs when a fuel undergoes oxidation • Oxidation: chemically reacting with oxygen – Gives off heat • Fire is a type of combustion • http: //www. youtube. com/watch? v=Uyg. Uc. Mk. Ry_c

COMBUSTION OF HYDROCARBONS Two Types: I. Complete Combustion • Occurs when there is plenty of oxygen • Carbon dioxide is one of the products hydrocarbon + oxygen carbon dioxide + water + heat Ex. ) Propane C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O + energy

II. Incomplete Combustion hydrocarbon + oxygen carbon monoxide + water + heat • Occurs when there is a shortage of oxygen • Carbon monoxide is one of the products. CH 4 + O 2 CO + 2 H 2 O + energy
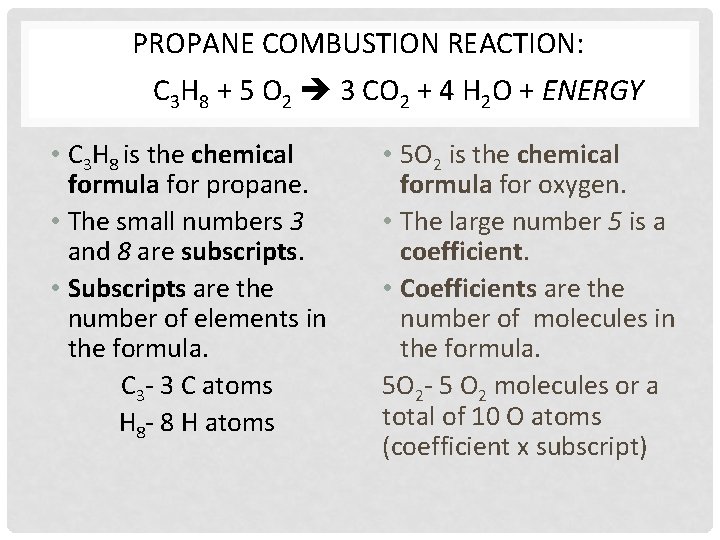
PROPANE COMBUSTION REACTION: C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O + ENERGY • C 3 H 8 is the chemical formula for propane. • The small numbers 3 and 8 are subscripts. • Subscripts are the number of elements in the formula. C 3 - 3 C atoms H 8 - 8 H atoms • 5 O 2 is the chemical formula for oxygen. • The large number 5 is a coefficient. • Coefficients are the number of molecules in the formula. 5 O 2 - 5 O 2 molecules or a total of 10 O atoms (coefficient x subscript)
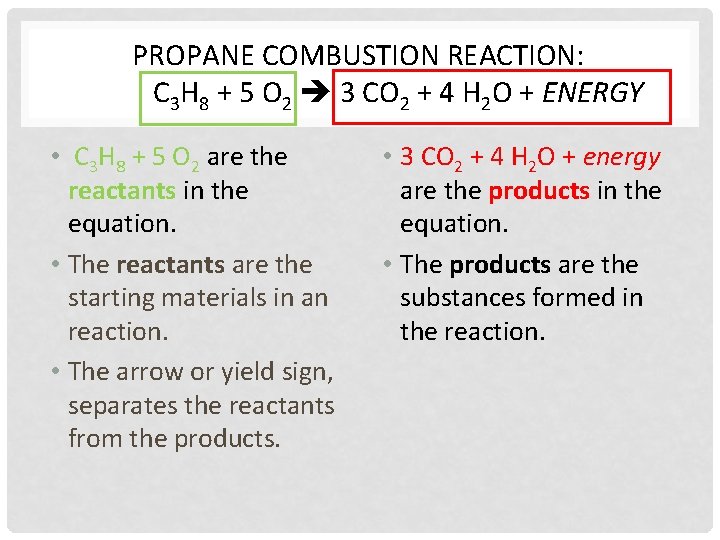
PROPANE COMBUSTION REACTION: C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O + ENERGY • C 3 H 8 + 5 O 2 are the reactants in the equation. • The reactants are the starting materials in an reaction. • The arrow or yield sign, separates the reactants from the products. • 3 CO 2 + 4 H 2 O + energy are the products in the equation. • The products are the substances formed in the reaction.

LAW OF CONSERVATION OF MASS • Atoms are never lost or gained in a chemical reaction • Just rearranged • Balanced equations, even on both sides, account for the conservation of mass. • The law of conservation of mass states that mass cannot be created or destroyed in ordinary chemical reactions and physical changes.

LETS PRACTICE! • Calculate the number of atoms and molecules for each chemical formula. C 3 H 8 5 O 2 3 CO 2 4 H 2 O
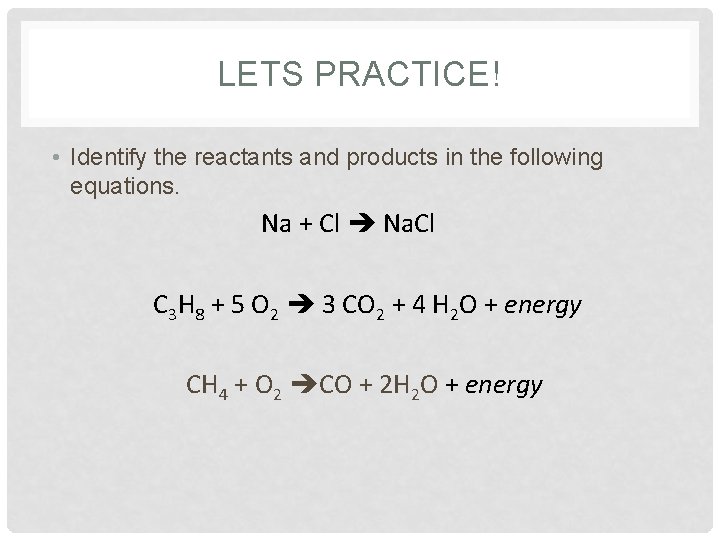
LETS PRACTICE! • Identify the reactants and products in the following equations. Na + Cl Na. Cl C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O + energy CH 4 + O 2 CO + 2 H 2 O + energy
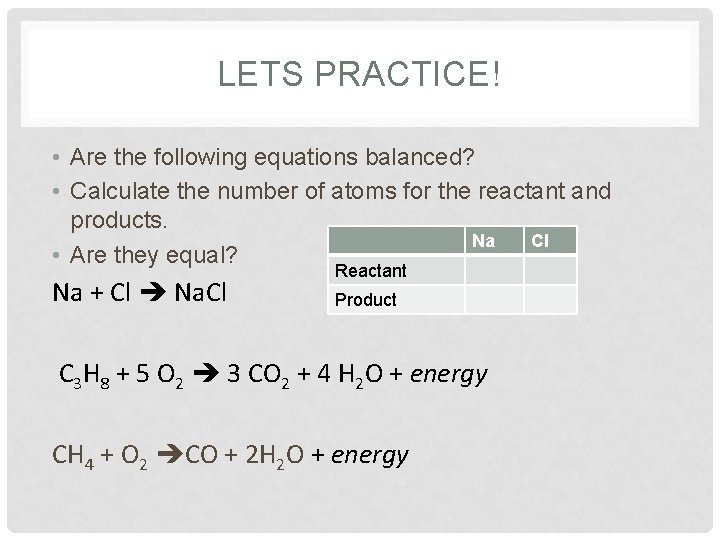
LETS PRACTICE! • Are the following equations balanced? • Calculate the number of atoms for the reactant and products. Na Cl • Are they equal? Na + Cl Na. Cl Reactant Product C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O + energy CH 4 + O 2 CO + 2 H 2 O + energy
- Slides: 16