Chemical Reactions and Chemical Equations An equation represents



- Slides: 12

Chemical Reactions and Chemical Equations An equation represents a reaction or chemical change

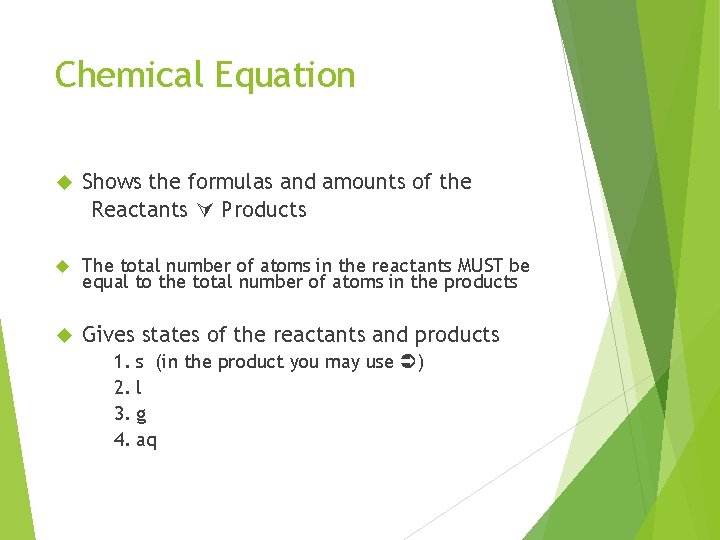
Chemical Equation Shows the formulas and amounts of the Reactants Products The total number of atoms in the reactants MUST be equal to the total number of atoms in the products Gives states of the reactants and products 1. 2. 3. 4. s (in the product you may use ) l g aq

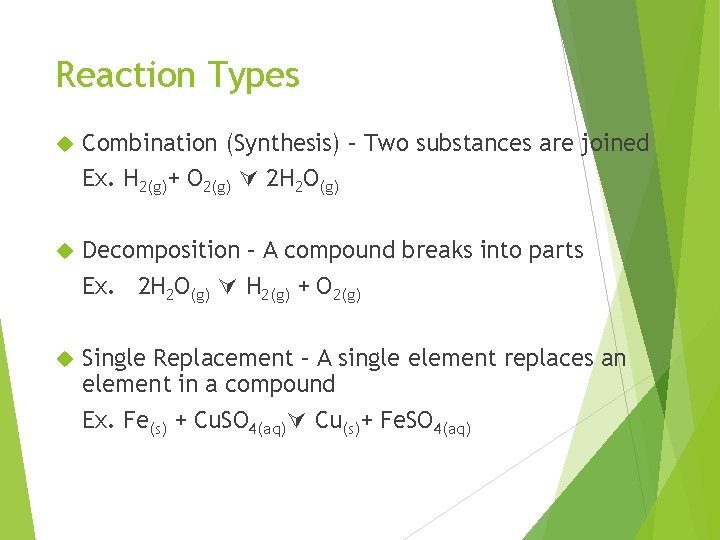
Reaction Types Combination (Synthesis) – Two substances are joined Ex. H 2(g)+ O 2(g) 2 H 2 O(g) Decomposition – A compound breaks into parts Ex. 2 H 2 O(g) H 2(g) + O 2(g) Single Replacement – A single element replaces an element in a compound Ex. Fe(s) + Cu. SO 4(aq) Cu(s)+ Fe. SO 4(aq)

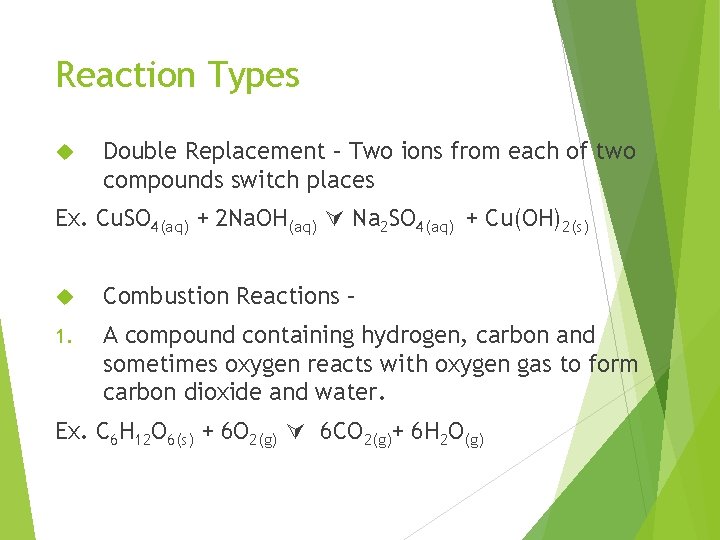
Reaction Types Double Replacement – Two ions from each of two compounds switch places Ex. Cu. SO 4(aq) + 2 Na. OH(aq) Na 2 SO 4(aq) + Cu(OH)2(s) Combustion Reactions – 1. A compound containing hydrogen, carbon and sometimes oxygen reacts with oxygen gas to form carbon dioxide and water. Ex. C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g)+ 6 H 2 O(g)

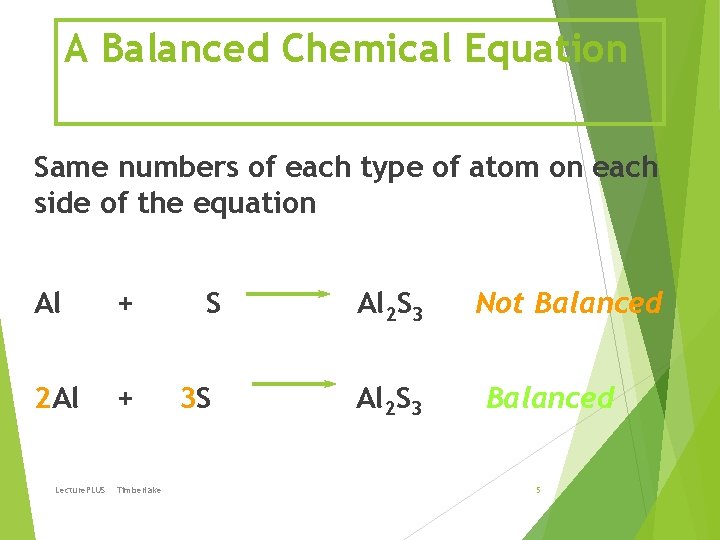
A Balanced Chemical Equation Same numbers of each type of atom on each side of the equation Al + 2 Al + Lecture. PLUS Timberlake S 3 S Al 2 S 3 Not Balanced 5

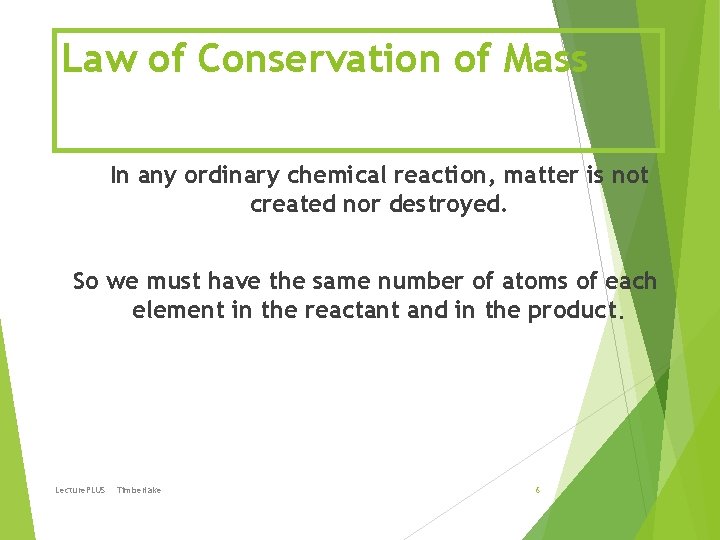
Law of Conservation of Mass In any ordinary chemical reaction, matter is not created nor destroyed. So we must have the same number of atoms of each element in the reactant and in the product. Lecture. PLUS Timberlake 6

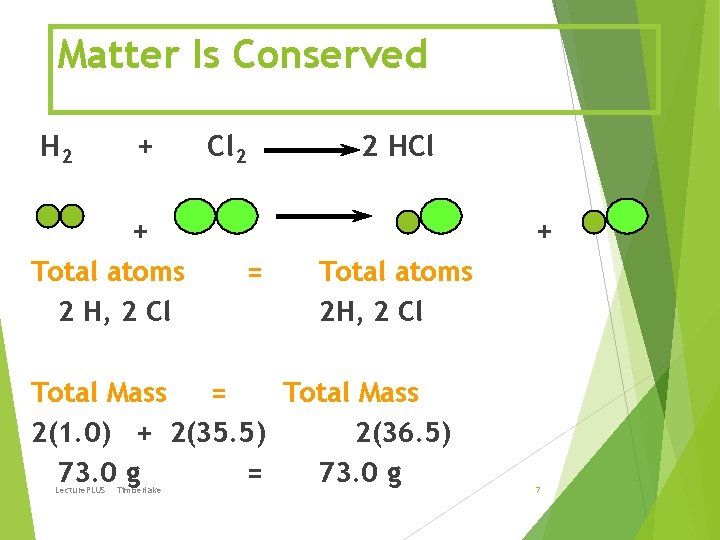
Matter Is Conserved H 2 + Cl 2 2 HCl + Total atoms 2 H, 2 Cl + = Total atoms 2 H, 2 Cl Total Mass = Total Mass 2(1. 0) + 2(35. 5) 2(36. 5) 73. 0 g = 73. 0 g Lecture. PLUS Timberlake 7

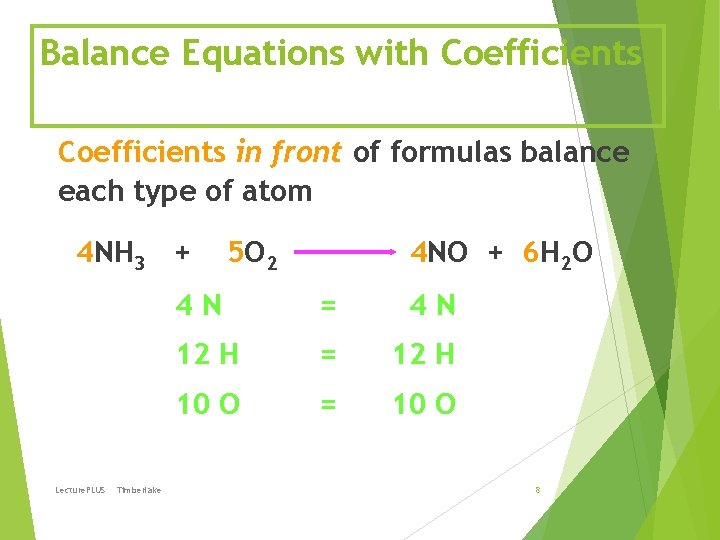
Balance Equations with Coefficients in front of formulas balance each type of atom 4 NH 3 + Lecture. PLUS Timberlake 5 O 2 4 NO + 6 H 2 O 4 N = 4 N 12 H = 12 H 10 O = 10 O 8

Total Ionic Equations Once you write the molecular equation (synthesis, decomposition, etc. ), you should check for reactants and products that are soluble or insoluble. We usually assume the reaction is in water We can use a solubility table to tell us what compounds dissolve in water. If the compound is soluble (does dissolve in water), then splits the compound into its component ions. If the compound is insoluble (does NOT dissolve in water), then it remains as a compound.

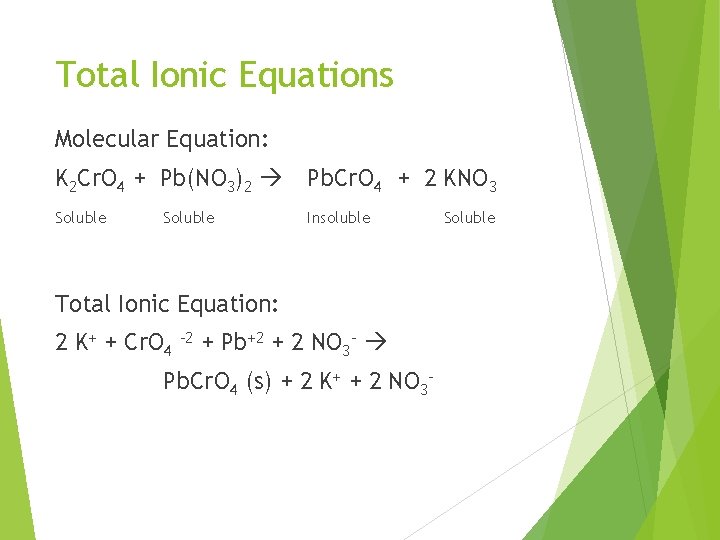
Total Ionic Equations Molecular Equation: K 2 Cr. O 4 + Pb(NO 3)2 Pb. Cr. O 4 + 2 KNO 3 Soluble Insoluble Soluble Total Ionic Equation: 2 K+ + Cr. O 4 -2 + Pb+2 + 2 NO 3 - Pb. Cr. O 4 (s) + 2 K+ + 2 NO 3 - Soluble

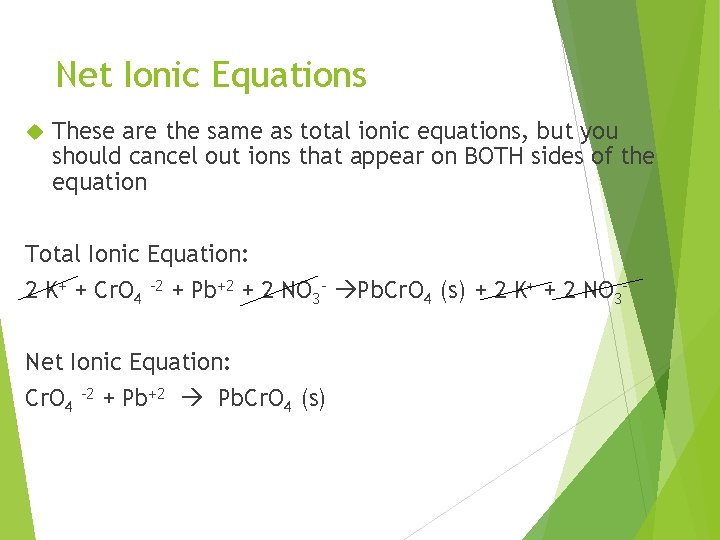
Net Ionic Equations These are the same as total ionic equations, but you should cancel out ions that appear on BOTH sides of the equation Total Ionic Equation: 2 K+ + Cr. O 4 -2 + Pb+2 + 2 NO 3 - Pb. Cr. O 4 (s) + 2 K+ + 2 NO 3 Net Ionic Equation: Cr. O 4 -2 + Pb+2 Pb. Cr. O 4 (s)

Net Ionic Equations Try this one! Write the molecular, total ionic, and net ionic equations for this reaction: Silver nitrate reacts with Lead (II) Chloride in hot water. Molecular: Total Ionic: Net Ionic: