Chemical Reactions 1 Types of Reactions 2 Indications


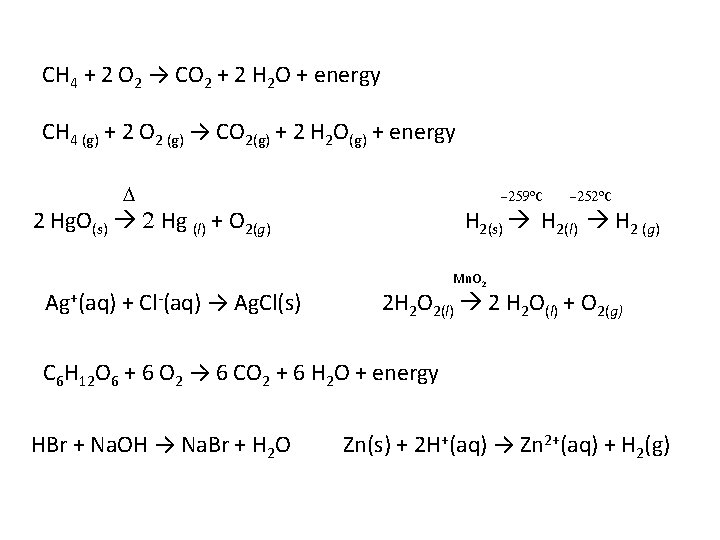

- Slides: 12

Chemical Reactions 1. Types of Reactions 2. Indications of Reactions 3. Relevance of Chemical Equations

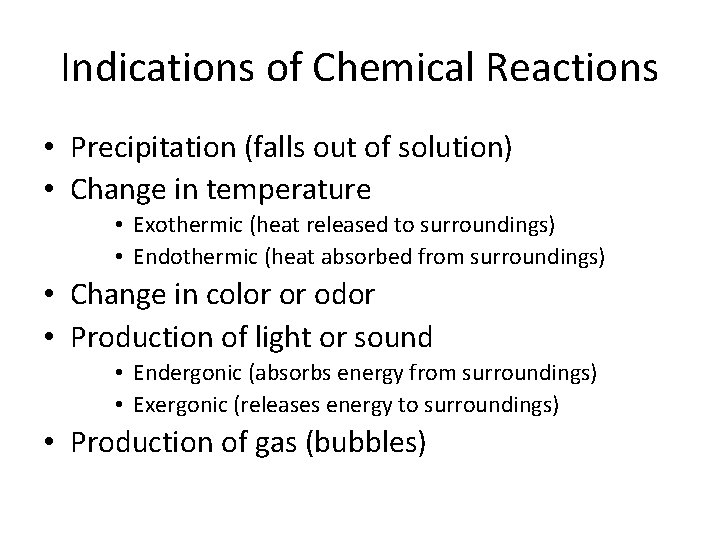
Indications of Chemical Reactions • Precipitation (falls out of solution) • Change in temperature • Exothermic (heat released to surroundings) • Endothermic (heat absorbed from surroundings) • Change in color or odor • Production of light or sound • Endergonic (absorbs energy from surroundings) • Exergonic (releases energy to surroundings) • Production of gas (bubbles)


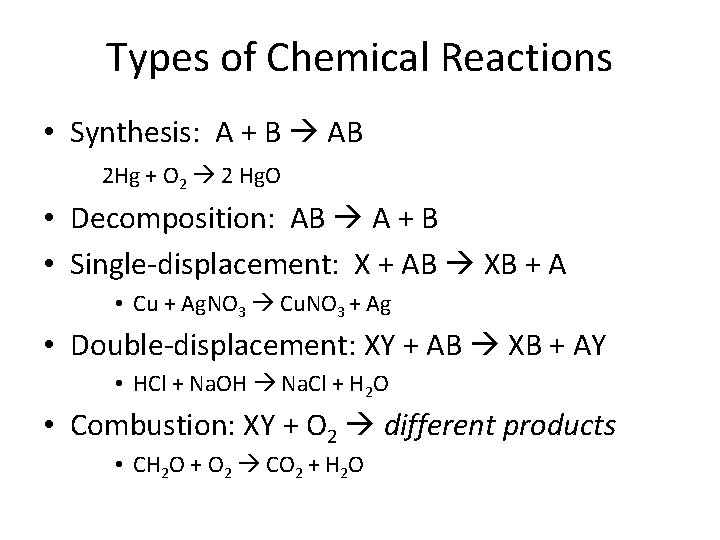
Types of Chemical Reactions • Synthesis: A + B AB 2 Hg + O 2 2 Hg. O • Decomposition: AB A + B • Single-displacement: X + AB XB + A • Cu + Ag. NO 3 Cu. NO 3 + Ag • Double-displacement: XY + AB XB + AY • HCl + Na. OH Na. Cl + H 2 O • Combustion: XY + O 2 different products • CH 2 O + O 2 CO 2 + H 2 O

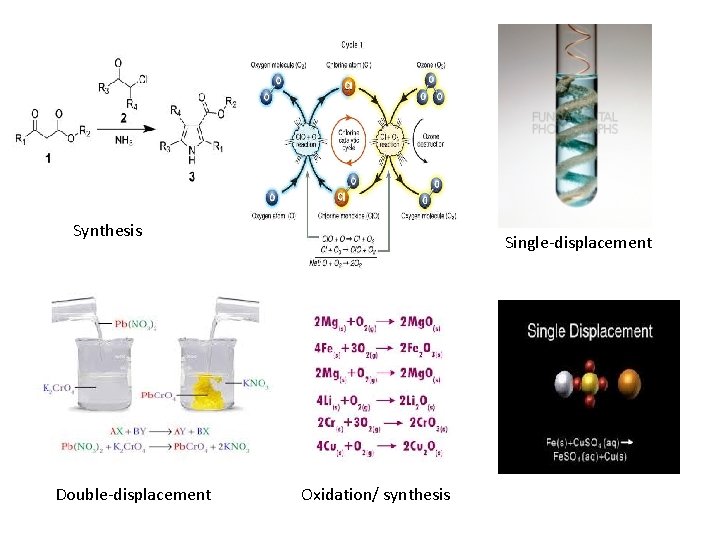
Synthesis Double-displacement Single-displacement Oxidation/ synthesis
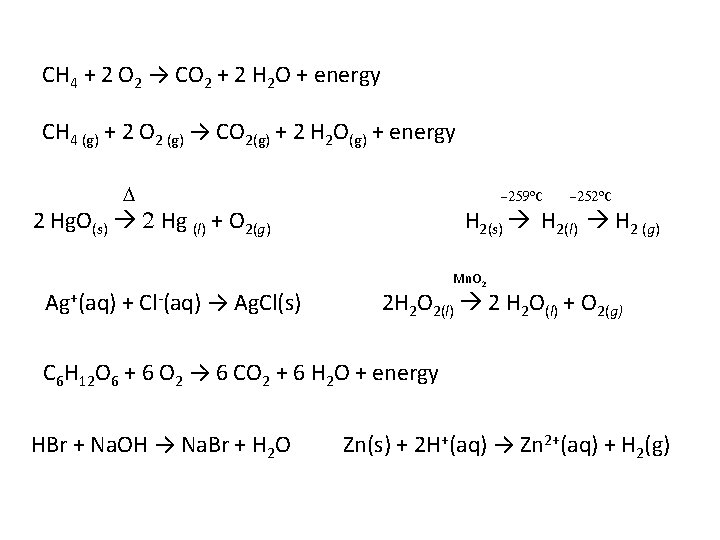
CH 4 + 2 O 2 → CO 2 + 2 H 2 O + energy CH 4 (g) + 2 O 2 (g) → CO 2(g) + 2 H 2 O(g) + energy D -259 o. C 2 Hg. O(s) 2 Hg (l) + O 2(g) Ag+(aq) + Cl-(aq) → Ag. Cl(s) -252 o. C H 2(s) H 2(l) H 2 (g) Mn. O 2 2 H 2 O 2(l) 2 H 2 O(l) + O 2(g) C 6 H 12 O 6 + 6 O 2 → 6 CO 2 + 6 H 2 O + energy HBr + Na. OH → Na. Br + H 2 O Zn(s) + 2 H+(aq) → Zn 2+(aq) + H 2(g)

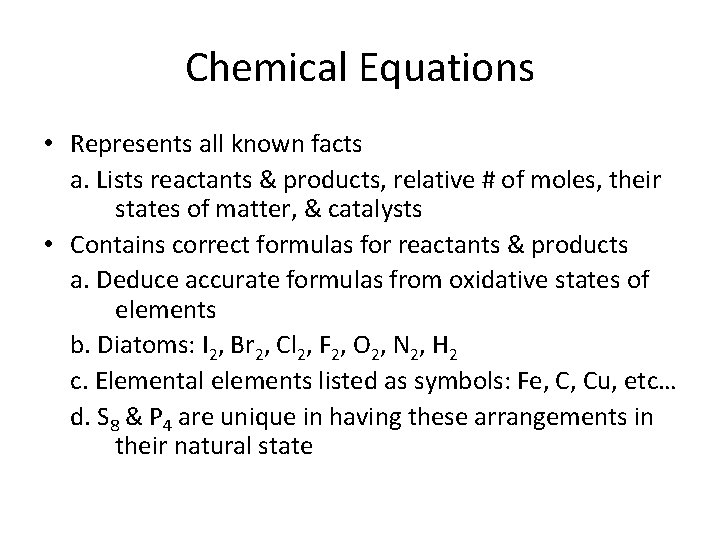
Chemical Equations • Represents all known facts a. Lists reactants & products, relative # of moles, their states of matter, & catalysts • Contains correct formulas for reactants & products a. Deduce accurate formulas from oxidative states of elements b. Diatoms: I 2, Br 2, Cl 2, F 2, O 2, N 2, H 2 c. Elemental elements listed as symbols: Fe, C, Cu, etc… d. S 8 & P 4 are unique in having these arrangements in their natural state

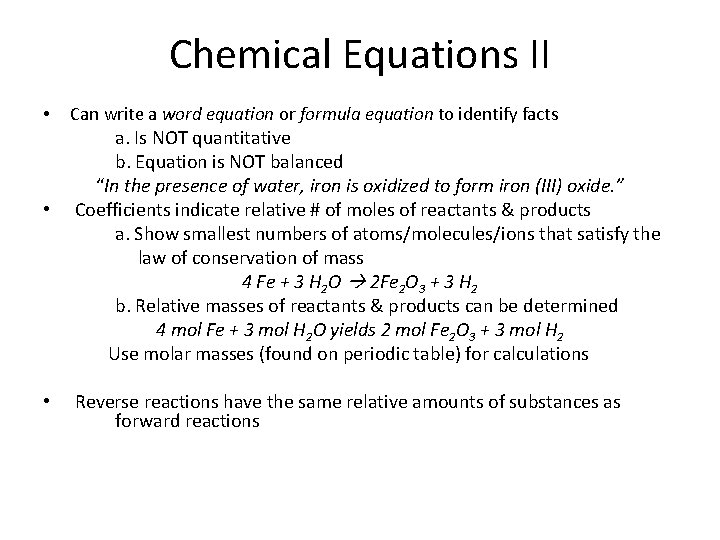
Chemical Equations II • • • Can write a word equation or formula equation to identify facts a. Is NOT quantitative b. Equation is NOT balanced “In the presence of water, iron is oxidized to form iron (III) oxide. ” Coefficients indicate relative # of moles of reactants & products a. Show smallest numbers of atoms/molecules/ions that satisfy the law of conservation of mass 4 Fe + 3 H 2 O 2 Fe 2 O 3 + 3 H 2 b. Relative masses of reactants & products can be determined 4 mol Fe + 3 mol H 2 O yields 2 mol Fe 2 O 3 + 3 mol H 2 Use molar masses (found on periodic table) for calculations Reverse reactions have the same relative amounts of substances as forward reactions

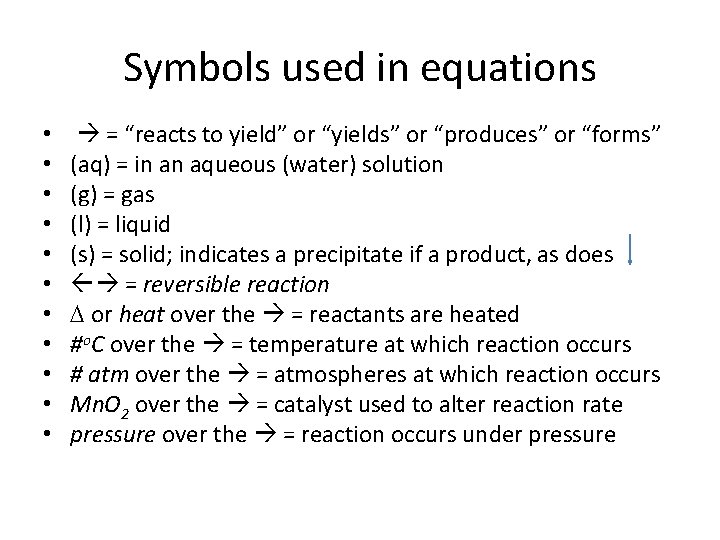
Symbols used in equations • • • = “reacts to yield” or “yields” or “produces” or “forms” (aq) = in an aqueous (water) solution (g) = gas (l) = liquid (s) = solid; indicates a precipitate if a product, as does = reversible reaction D or heat over the = reactants are heated #o. C over the = temperature at which reaction occurs # atm over the = atmospheres at which reaction occurs Mn. O 2 over the = catalyst used to alter reaction rate pressure over the = reaction occurs under pressure

Balancing Chemical Equations • Balance equation according to law of conservation of mass – One can only change coefficients, NEVER subscripts – Balance different types of atoms one at a time – Balance those in compounds 1 st – Balance polyatomic ions that appear on both sides of the equation – Balance free H & O atoms last. • ALWAYS do a final count of all atoms to ensure equation is balanced.

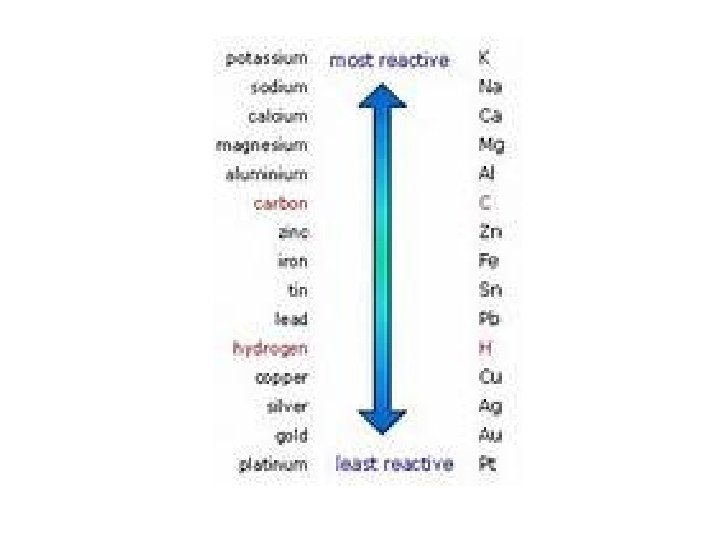
Activity Series of Metals • Most reactive metal at top of list can replace those below it. • Reactions based on empirical data • Chemical equations don’t always relate to real-world occurrences. • Timing is everything. (It may happen so slowly that you don’t live long enough to see it. ) • Temperature may be a factor.

 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Indications of chemical reactions
Indications of chemical reactions Indications of chemical reactions
Indications of chemical reactions Types of reactions
Types of reactions Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Different types of redox reactions
Different types of redox reactions Types of reactions
Types of reactions 4 types of chemical reactions
4 types of chemical reactions Types of reaction
Types of reaction 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions