Chemical Reactions 1 All chemical reactions have two




























- Slides: 46

Chemical Reactions 1

All chemical reactions have two parts l Reactants - the substances you start with l Products- the substances you end up with l The reactants turn into the products. l Reactants ® Products l 2

In a chemical reaction The way atoms are joined is changed l Atoms aren’t created or destroyed. l Can be described several ways l In a sentence l Copper reacts with chlorine to form copper (II) chloride. l In a word equation l Copper + chlorine ® copper (II) chloride l 3

Symbols used in equations the arrow separates the reactants from the products l Read “reacts to form” l The plus sign = “and” l (s) after the formula –solid (sometimes could say (cr)-crystal l (g) after the formula -gas l (l) after the formula -liquid l 4

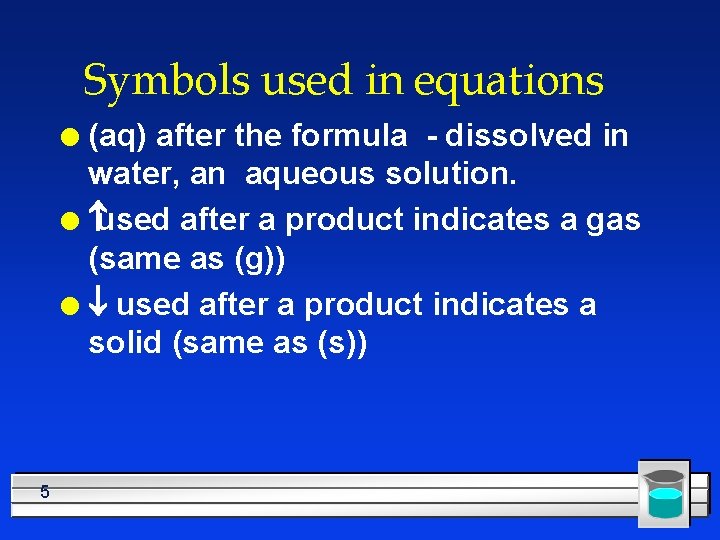
Symbols used in equations (aq) after the formula - dissolved in water, an aqueous solution. l used after a product indicates a gas (same as (g)) l ¯ used after a product indicates a solid (same as (s)) l 5

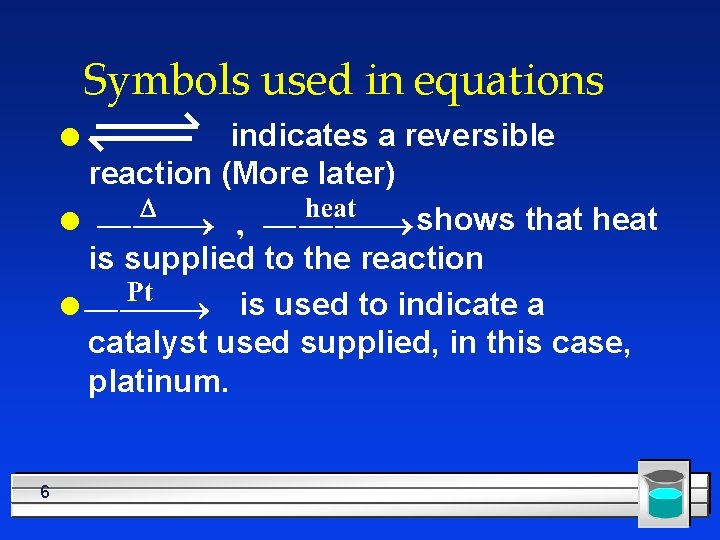
Symbols used in equations indicates a reversible reaction (More later) l shows that heat is supplied to the reaction l is used to indicate a catalyst used supplied, in this case, platinum. l 6

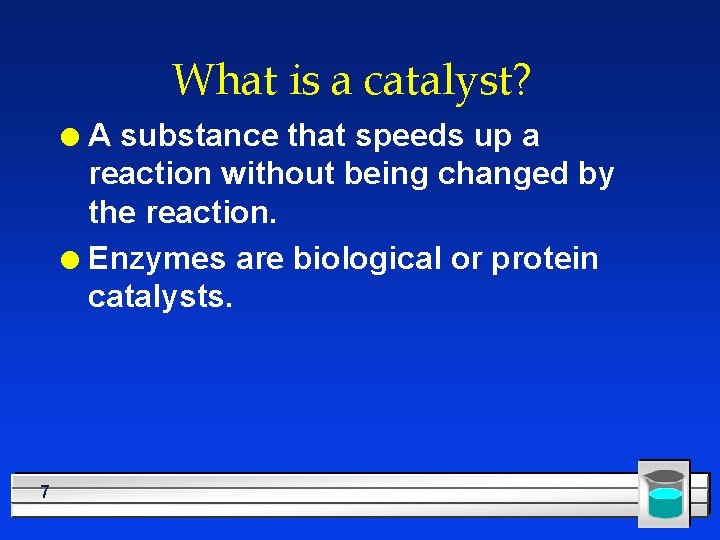
What is a catalyst? A substance that speeds up a reaction without being changed by the reaction. l Enzymes are biological or protein catalysts. l 7

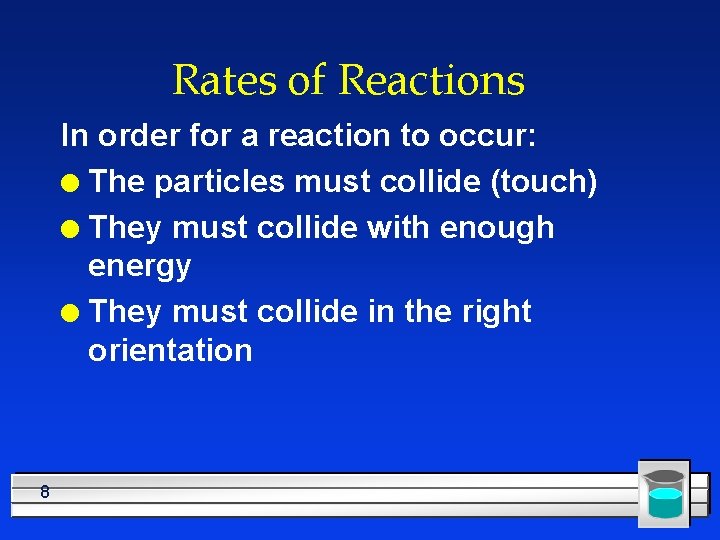
Rates of Reactions In order for a reaction to occur: l The particles must collide (touch) l They must collide with enough energy l They must collide in the right orientation 8

Factors that affect reaction rate: Temperature (particle energy) l Particle size l Surface area l Particle contact (stirring) l 9

Balancing Chemical Equations 10

Balanced Equation Atoms can’t be created or destroyed l All the atoms we start with we must end up with l A balanced equation has the same number of each element on both sides of the equation. l 11

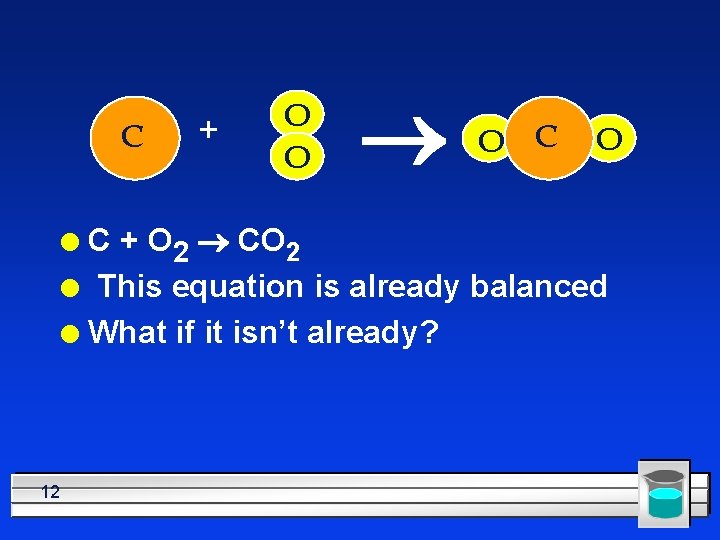
C + O O ® O C + O 2 ® CO 2 l This equation is already balanced l What if it isn’t already? l 12

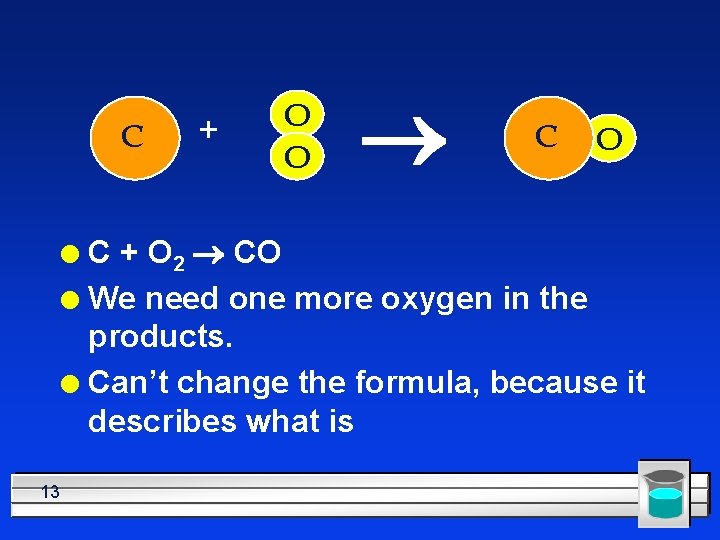
C + O O ® C O C + O 2 ® CO l We need one more oxygen in the products. l Can’t change the formula, because it describes what is l 13

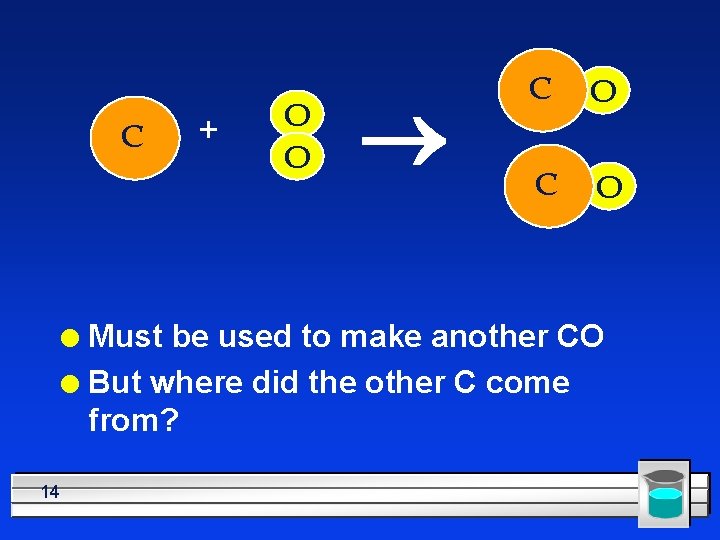
C + O O ® C O Must be used to make another CO l But where did the other C come from? l 14

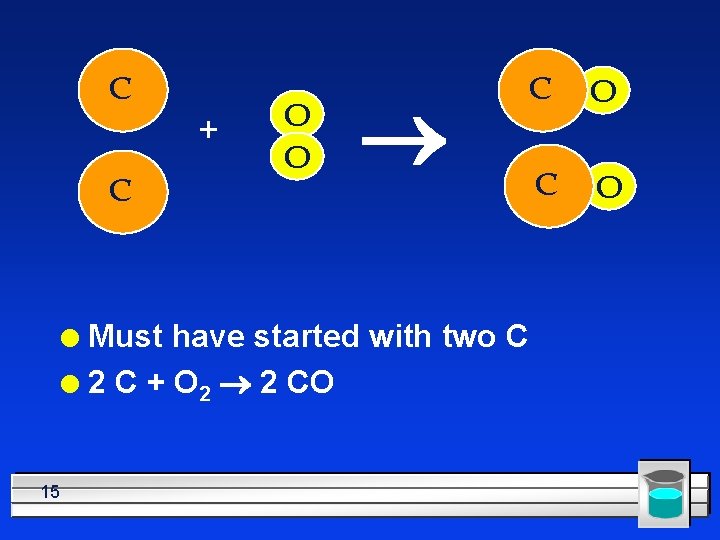
C + C O O ® Must have started with two C l 2 C + O 2 ® 2 CO l 15 C O

Rules for balancing 1 Write the correct formulas for all the reactants and products 2 Count the number of atoms of each type appearing on both sides 3 Balance the elements one at a time by adding coefficients (the numbers in front) 4 Check to make sure it is balanced. 16

Never Change a subscript to balance an equation. l If you change the formula you are describing a different reaction. l H 2 O is a different compound than H 2 O 2 l Never put a coefficient in the middle of a formula l 2 Na. Cl is okay, Na 2 Cl is not. l 17

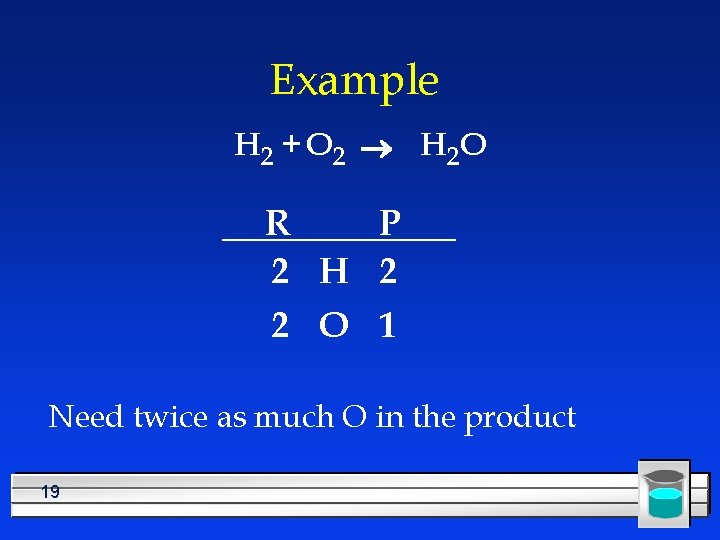
Example H 2 + O 2 ® H 2 O Make a table to keep track of where you are at 18

Example H 2 + O 2 ® H 2 O R P 2 H 2 2 O 1 Need twice as much O in the product 19

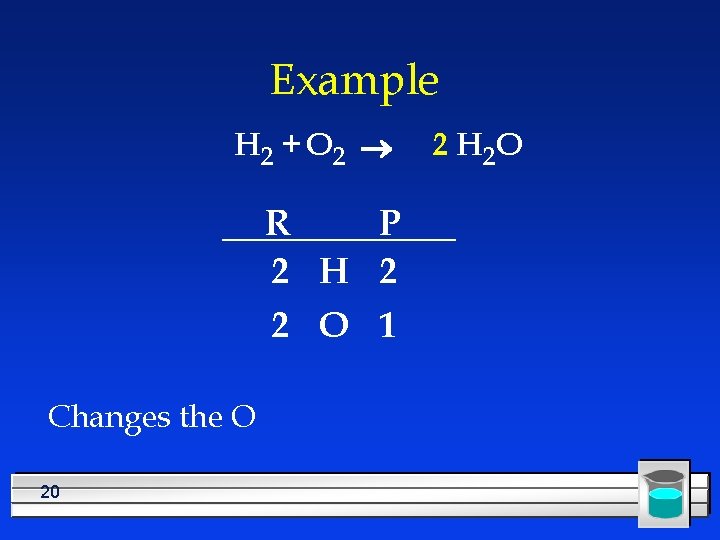
Example H 2 + O 2 ® R P 2 H 2 2 O 1 Changes the O 20 2 H 2 O

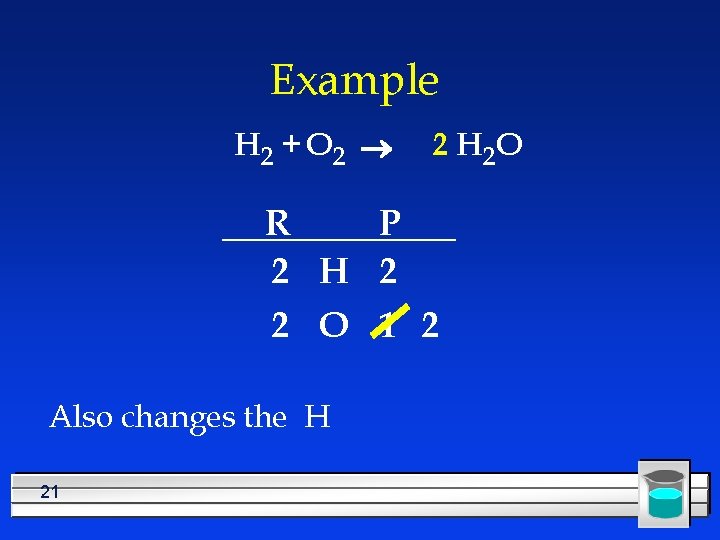
Example H 2 + O 2 ® 2 H 2 O R P 2 H 2 2 O 1 2 Also changes the H 21

Example H 2 + O 2 ® 2 H 2 O R P 2 H 2 4 2 O 1 2 Need twice as much H in the reactant 22

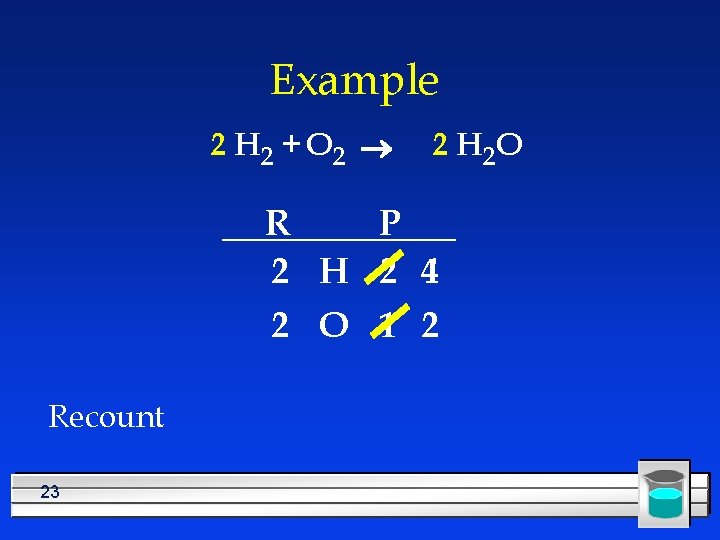
Example 2 H 2 + O 2 ® 2 H 2 O R P 2 H 2 4 2 O 1 2 Recount 23

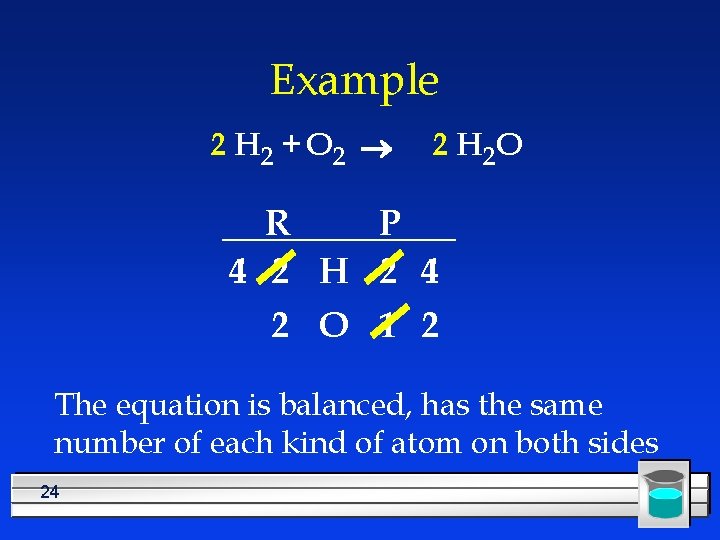
Example 2 H 2 + O 2 ® 2 H 2 O R P 4 2 H 2 4 2 O 1 2 The equation is balanced, has the same number of each kind of atom on both sides 24

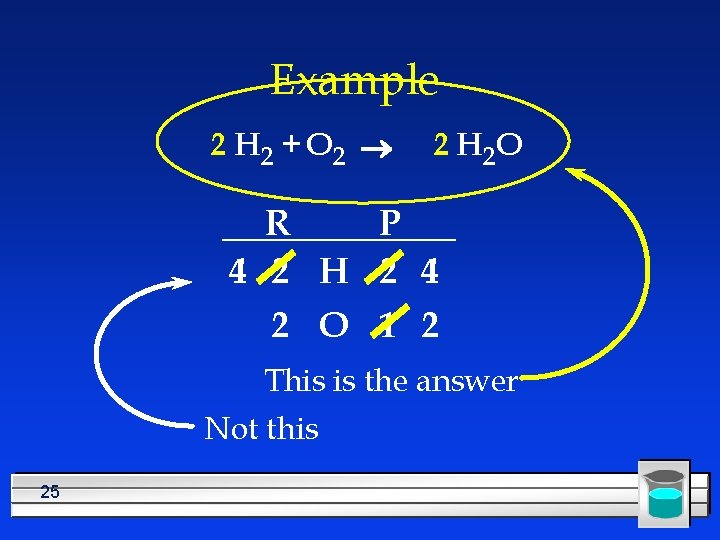
Example 2 H 2 + O 2 ® 2 H 2 O R P 4 2 H 2 4 2 O 1 2 This is the answer Not this 25

Examples Ag. NO 3 + Cu ® Cu(NO 3)2 + Ag l Mg + N 2 ® Mg 3 N 2 l P + O 2 ® P 4 O 10 l Na + H 2 O ® H 2 + Na. OH l CH 4 + O 2 ® CO 2 + H 2 O l 26

Evidence of Reactions Looking for the clues 27

Evidence of Reactions Just because the evidence is there DOES NOT mean a chemical reaction is taking place Have to look at everything that is going on 28

Evidence of Reactions 1. Formation of a gas Observations: Bubbles Smoke Odor/fumes 29

Evidence of Reactions 2. Change in color Be Careful! Some changes in color are physical changes! 30

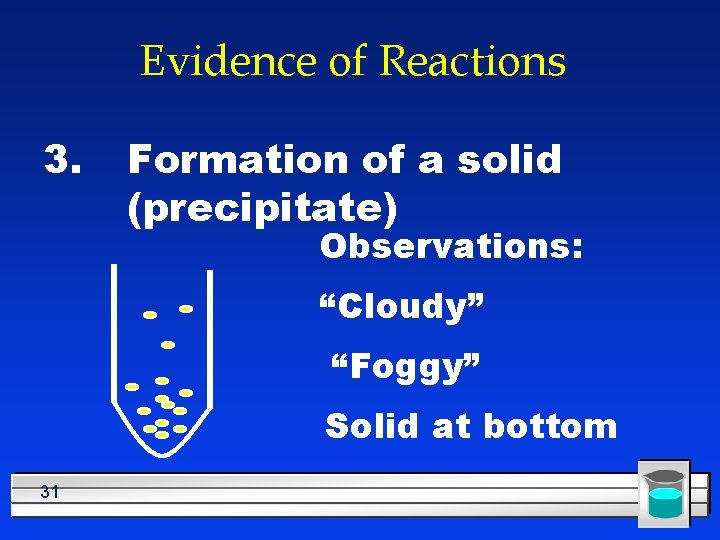
Evidence of Reactions 3. Formation of a solid (precipitate) Observations: “Cloudy” “Foggy” Solid at bottom 31

Evidence of Reactions 4. Change in heat or light energy Observations: gets warm/hot/cold spark or explosion glows 32

Exothermic Reactions: Give off energy as heat or light WHY? ? Because energy stored in chemical BONDS In EXOthermic reactions there is MORE energy stored in bonds of reactants than needed to form products! So. . there is left over energy! 33

Endothermic Reactions: Absorb energy as heat or light WHY? ? Because the energy stored in the bonds of the reactants is NOT ENOUGH to hold together the products. MORE ENERGY IS NEEDED! 34

Evidence of Reactions Summary 1. 2. 3. Formation of a gas Change in color Formation of a solid (precipitate) 4. Change in heat or light energy 35

Types of Reactions Predicting the Products 36

Types of Reactions There are millions of reactions. l Can’t remember them all l Fall into several categories. l We will learn 5 types. l Will be able to predict the products. l For some we will be able to predict whether they will happen at all. l Will recognize them by the reactants l 37

#1 Combination Reactions Combine - put together l 2 elements, or compounds combine to make one compound. l Ca +O 2 ® Ca. O l SO 3 + H 2 O ® H 2 SO 4 l We can predict the products l Mg + N 2 ® l 38

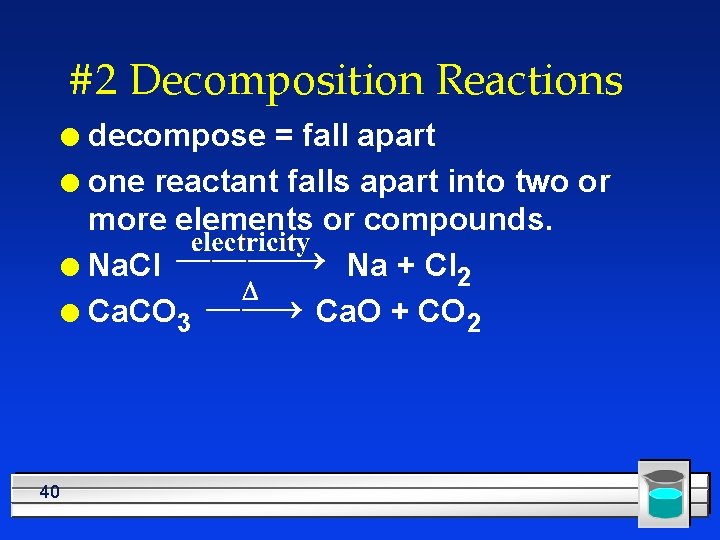
#2 Decomposition Reactions decompose = fall apart l one reactant falls apart into two or more elements or compounds. l Na. Cl Na + Cl 2 l Ca. CO 3 Ca. O + CO 2 l 40

#3 Single Replacement One element replaces another l Reactants must be an element and a compound. l Products will be a different element and a different compound. l Na + KCl ® K + Na. Cl l F 2 + Li. Cl ® Li. F + Cl 2 l 43

#3 Single Replacement Metals replace metals (and hydrogen) l K + Al. N ® l Zn + HCl ® l Think of water as HOH l Metals replace one of the H, combine with hydroxide. l Na + HOH ® l 44

#3 Single Replacement We can tell whether a reaction will happen l Some are more active than other l More active replaces less active l Higher on the list replaces lower. l If the element by itself is higher, it happens, in lower it doesn’t l 45

#3 Single Replacement Nonmetals can replace other nonmetals l Limited to F 2 , Cl 2 , Br 2 , I 2 l The order of activity is that on the table. l Higher replaces lower. l F 2 + HCl ® l Br 2 + KCl ® l 47

#4 Double Replacement Two things replace each other. l Reactants must be two ionic compounds or acids. l Usually in aqueous solution l Na. OH + Fe. Cl 3 ® l The positive ions change place. l Na. OH + Fe. Cl 3 ® Fe+3 OH- + Na+1 Cl-1 l Na. OH + Fe. Cl 3 ® Fe(OH)3 + Na. Cl l 48

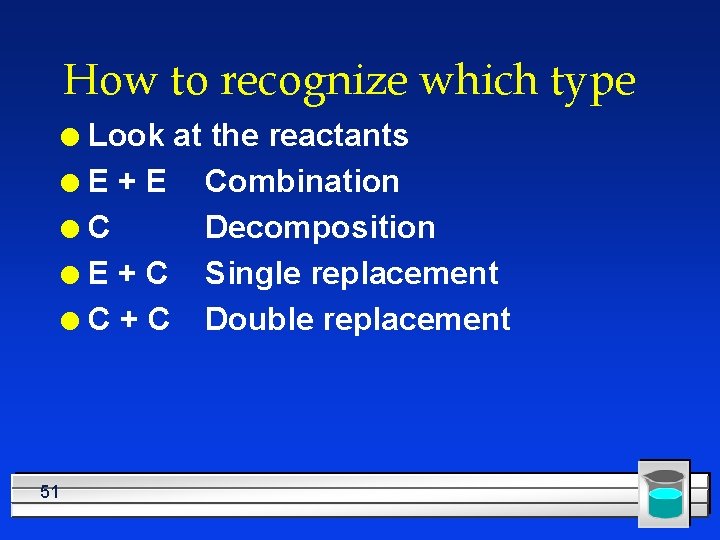
How to recognize which type Look at the reactants l. E+E Combination l. C Decomposition l E + C Single replacement l C + C Double replacement l 51

Last Type Combustion of hydrocarbons l A compound composed of only C H and maybe O is reacted with oxygen l If the combustion is complete, the products will be CO 2 and H 2 O. l If the combustion is incomplete, the products will be CO and H 2 O. l 53
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Are all chemical reactions reversible
Are all chemical reactions reversible Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Name all rays
Name all rays How to write redox half reactions
How to write redox half reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet 8 vertices 12 edges
8 vertices 12 edges We all have two lives
We all have two lives When two curves coincide the two objects have the same
When two curves coincide the two objects have the same Example of neutralization reaction
Example of neutralization reaction Stoichiometry island diagram
Stoichiometry island diagram Examples of chemical change
Examples of chemical change Types of chemical reactions redox
Types of chemical reactions redox Types of reaction
Types of reaction 4 types of chemical reactions
4 types of chemical reactions Tyoes of chemical reactions
Tyoes of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions
Chapter 10 chemical reactions The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Ouchterlony
Ouchterlony Predicting products of chemical reactions
Predicting products of chemical reactions More predicting products of chemical reactions
More predicting products of chemical reactions Section 3 predicting the products of chemical reactions
Section 3 predicting the products of chemical reactions Unit 11 chemical reactions
Unit 11 chemical reactions Lesson 68 toxic reactions chemical equations
Lesson 68 toxic reactions chemical equations Four types of chemical reactions
Four types of chemical reactions Biology-roots.com
Biology-roots.com Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life Five chemical
Five chemical Chemical reactions reactants and products
Chemical reactions reactants and products 5 general types of chemical reactions
5 general types of chemical reactions Solubility rules
Solubility rules Chapter 9 chemical reactions study guide
Chapter 9 chemical reactions study guide Rate constant and equilibrium constant
Rate constant and equilibrium constant Chapter 9 chemical reactions
Chapter 9 chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Four types of chemical reactions
Four types of chemical reactions Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions




































































