Chemical Reaction Predictions Types of Chemical Reactions 1








![What is the concentration ([ ]) of a particular ion in solution? Sometimes [ion] What is the concentration ([ ]) of a particular ion in solution? Sometimes [ion]](https://slidetodoc.com/presentation_image_h/ac30344558ddf78dc2e3f1a8f68c8159/image-9.jpg)








































- Slides: 53

Chemical Reaction Predictions

Types of Chemical Reactions 1) Single Displacement 2) Double Displacement 3) Decomposition 4) Synthesis 5) Combustion

Aqueous Solutions Compounds dissolved into water. Can contain molecules or ions in a solution. How do you distinguish between ion or molecule?

DISSOCIATION !! The ability of a compound to breakdown in a solution into individual ions Ionic Compounds Break down into cations and anions Electrical conductors—ions flow through solution Molecular Compound remains intact as “molecules, ” no breakdown Generally NOT electrical conductors

Nonelectrolyte NO dissociation of the compound into anions and cations Compound remains in its molecular form when dissolved in a solution No conduction of electricity Ex. Pure water, molecular compounds, organic compounds

Electrolytes Compound dissolved in a solution that breaks down into cations and anions. Dissociates into enough ions to conduct electricity. Strong vs. weak—dependent on the amount of ions in the solution

Strong Electrolyte LOTS of ions present in solution Light bulb burns brightly ! Complete dissociation of the compound into ions Good electrical conductor Ex. Na. Cl, all soluble ionic compounds, very few molecular compounds

Weak Electrolyte Compound is at a crossroads part dissociates into ions—partial ionization part still exists in the molecular form Some electrical conduction but poor Dimly lit light bulb Ex. Acetic acid, carboxylic acid/amines
![What is the concentration of a particular ion in solution Sometimes ion What is the concentration ([ ]) of a particular ion in solution? Sometimes [ion]](https://slidetodoc.com/presentation_image_h/ac30344558ddf78dc2e3f1a8f68c8159/image-9.jpg)
What is the concentration ([ ]) of a particular ion in solution? Sometimes [ion] = [compound], but not always An ion can have only ONE concentration in a solution May have multiple sources for one ion. What do you do then? (add them up)

Example 1: Hercules has obtained an aqueous solution of 0. 00384 M Na 2 SO 4 and 0. 00202 M Na. Cl. What is the concentration (in molarity) of each ion present in the solution? Total ion concentration?

Example 2: Oral Rehydration Therapy (ORT) is an electrolyte solution containing 3. 5 g Na. Cl, 1. 5 g KCl, 2. 9 g sodium citrate (Na 3 C 6 H 5 O 7) and 20. 0 g glucose (C 6 H 12 O 6) in 1 liter. What is the molarity of each ion/molecule in a solution of ORT? Hint: sodium citrate is a strong electrolyte, glucose is a nonelectrolyte

Solubility How much solute dissolves in a solution to produce a saturated solution Temperature and Pressure dependent Increase with increasing temperature Increases with decreasing temperature (ex. Water in lake) Pressure increases, solubility increases (ex. Soda can)

Types of Solutions 1) Saturated Maximum amount of solute that can be dissolved in a solvent Certain temperature and pressure 2) Unsaturated Solution with LESS solute than the maximum solute amount at a certain temperature and pressure More solute can be added and dissolved in the solution 3) Supersaturated Contains MORE solute than the maximum solute amount

Which compounds are soluble in water? 1) Ba. Cl 2 2) Pb (NO 3)2 3) Na 2 S 4) Ba. CO 3 5) Pb. S

AP Question Snow White conducts a complete combustion of a hydrocarbon with excess oxygen. This combustion produces equimolar quantities of carbon dioxide and water. What is a possible molecular formula for the compound? A) C 2 H 2 B) C 2 H 6 C) C 4 H 8 D) C 6 H 6

Reaction Predictions Precipitation Reactions

Precipitation Prediction 1) Write the reactants in ionic from breakdown into ionic form if compounds are soluble leave as molecules if insoluble 2) Determine the solubility of the products. Use solubility Rules

Precipitation Predictions (cont. ) 3) Check to see if one product is insoluble in water. Product will fall out of solution, identified as precipitate 4) Write the net ionic equation Displays which ions are directly involved in the reaction, produce the precipitate Ions existing on BOTH sides of the equation are “spectator ions” (do NOT participate in precipitate formation) Spectator ions are eliminated

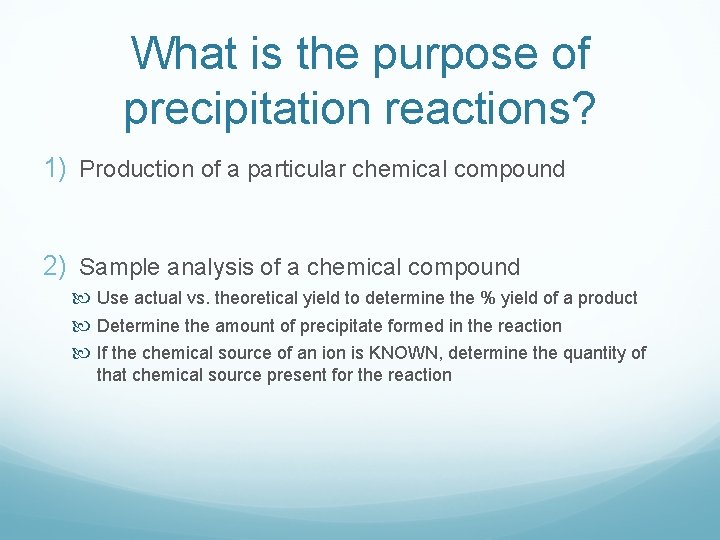
What is the purpose of precipitation reactions? 1) Production of a particular chemical compound 2) Sample analysis of a chemical compound Use actual vs. theoretical yield to determine the % yield of a product Determine the amount of precipitate formed in the reaction If the chemical source of an ion is KNOWN, determine the quantity of that chemical source present for the reaction

Example 1: A 240 g sample of chicken broth combined with excess Ag. NO 3 (aq) produces 4. 302 g Ag. Cl. If all Cl – ions originate from Na. Cl, how much salt is present in the chicken broth (in grams)?

Example 2: A white precipitate is formed when an unknown white solid is dissolved into a solution and reacted with Ba(NO 3)2. Arthur with the help of Merlin has narrowed the identity of the unknown solid to be 3 possibilities: Mg. Cl 2, Mg. SO 4, or Mg(NO 3)2. Identify the unknown.

Reaction Predictions Oxidation-Reduction (Redox) Reactions

Oxidation-Reaction? ? It’s more common than you think……. .

Oxidation Number Rules Priority 1 st, if there is ANY conflict—go with highest priority 1) Sum of oxidation numbers = 0 for ALL neutral compounds (atoms, molecules) Any single atoms are also assigned oxidation # = 0

Oxidation Number Rules 2) Sum of oxidation numbers for an ion = ion’s charge Examples: SO 4 -2 NH 4+ Al ion Cl ion

Oxidation Number Rules 3) Group 1 A metals = +1 Group 2 A metals = +2 Examples: Mg. SO 4 K 3 PO 4

Oxidation Number Rules 4) For the majority of chemical compounds— F = -1 H = +1 (sometimes will have -1) O = -2 Examples: NH 3 H 2 O CO HF KF

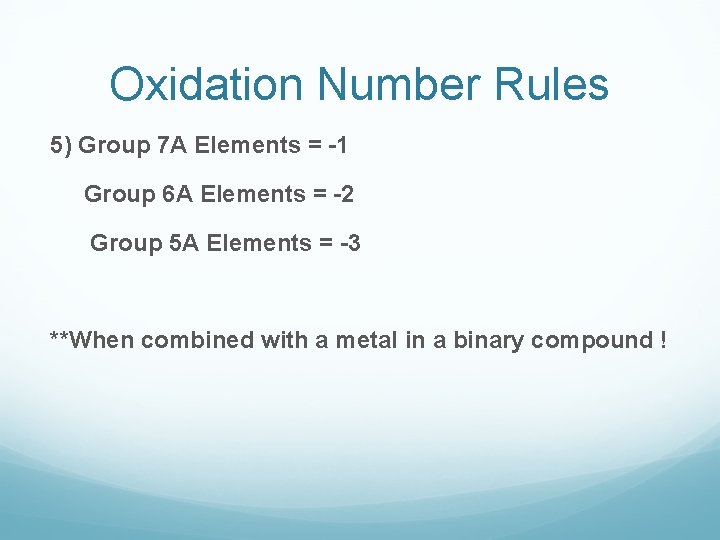
Oxidation Number Rules 5) Group 7 A Elements = -1 Group 6 A Elements = -2 Group 5 A Elements = -3 **When combined with a metal in a binary compound !

Oxidation-Reduction Reactions Electron transfer between ionic compounds, change in oxidation numbers One compound wants to GIVE electrons, other compound wants to TAKE electrons.

2 chemical processes happening at the same time ! 1) Reduction— GAIN of electrons in element Compound becomes more NEGATIVE DECREASE in oxidation number 2) Oxidation LOSS of electrons Compound becomes more POSITIVE INCREASE in oxidation number

Oxidation CANNOT happen without Reduction CANNOT happen without Oxidation Both have to happen in a redox reaction ! ! ! (electrons gained = electrons lost)

Oxidation Numbers/State Allows us to identify redox reactions A change in these numbers-----REDOX REACTION ! ! ! Acts like all chemical compounds are ionic----gives atom a charge it would have IF it was ionic

Example 2: 2 Na + Cl 2 2 Na. Cl Assign oxidation numbers Identify where oxidation occurs, where reduction occurs.

Reaction Predictions Acids and Bases

1) Strong Acids Completely dissociate into ions in an aqueous solution, only a few acids Strong electrolytes [acid] = [ion concentration] in solution, no molecules left HCl, HBr, HI, HNO 3, H 2 SO 4, HCl. O 4 (KNOW THEM!!!) Ex. HBr

2) Weak Acids Partially dissociate in an aqueous solution Weak electrolytes Some molecules break up into ions, other molecules stay together in solution [weak acid] ≠ [ion] Ex. CH 3 COOH

Multiple Ionizations Some acids can donate more than one H+ ion and go through multiple ionizations H 3 PO 4 and H 2 SO 4

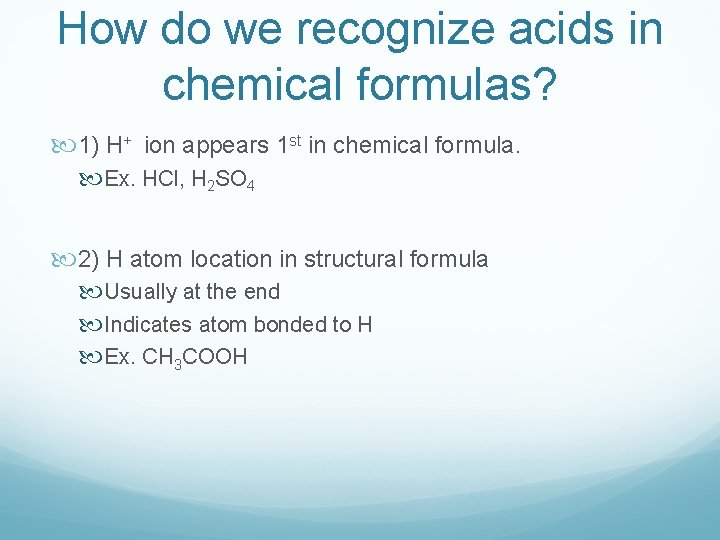
How do we recognize acids in chemical formulas? 1) H+ ion appears 1 st in chemical formula. Ex. HCl, H 2 SO 4 2) H atom location in structural formula Usually at the end Indicates atom bonded to H Ex. CH 3 COOH

1) Strong Bases Completely dissociate into ions in aqueous solutions Strong electrolytes, mainly ionic compounds [strong base] = [ion] in solution Most Group IA and IIA metals combined with hydroxide ion Li. OH, Na. OH, KOH, Rb. OH, Cs. OH, Mg(OH)2 , Ca(OH)2 , Sr(OH)2 , Ba(OH)2 –KNOW THEM ! ! !

2) Weak Bases Partially dissociate in an aqueous solution Weak electrolytes, typically molecular compounds Some molecules break up into ions, other molecules stay together in solution [weak base] ≠ [ion] Do not necessarily have hydroxide (OH-) ion in chemical compound React with water and produce the hydroxide ion as a product.

How do we recognize bases in a chemical formula? 1) Ionic Compound with metal cation + hydroxide (OH-) anion Strong base 2) Molecular compound has OH covalently bound, not existing as OH- ion Consisting of nonmetals Rely on chemical equation to show ionization Most common weak bases: ammonia (NH 3) and amines (R-NH 2)

Neutralization Reactions Acids and bases cancel each other out, “neutralize” or even out the solution ACID + BASE SALT + H 2 O How do we determine when a solution is neutral? Check p. H with litmus paper, color changes Acid/base indicator– compound where its color is influenced by the [H+] and [OH-] in solution

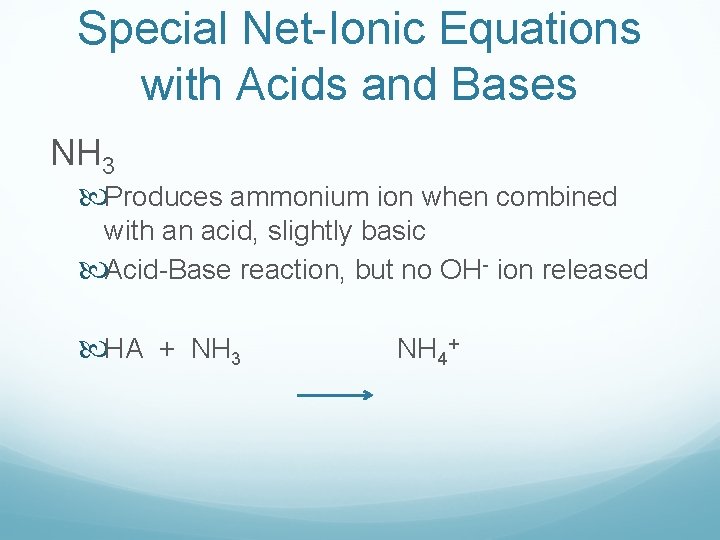
Special Net-Ionic Equations with Acids and Bases NH 3 Produces ammonium ion when combined with an acid, slightly basic Acid-Base reaction, but no OH- ion released HA + NH 3 NH 4+

Balancing Redox Reactions Acidic Solutions

Half-Reaction Procedure for Balancing 1) Write the half-reaction equations (oxidation and reduction). 2) Balance the half-reactions normally based on atom amount. Balance O and H atoms last. 3) Balance electric charges Add electrons to each side when necessary to allow the charges to equal on both sides of the equation. Reduction—electrons gained, write on reactant side Oxidation—electrons lost/releasing electrons, write on product side

Procedure (cont. ) 4) Balance electrons in the half-reactions with coefficients. Multiply one or both half-reactions if necessary 5) Add half-reactions. 6) Simplify the overall redox reaction. Divide coefficients if necessary

How do we recognize redox reactions occurring in acidic solutions? H 2 O or H+ ions are present as reactants or products H 2 O or H+ ions found in half-reactions and in the final balanced equation. Add H 2 O molecules when needed to balance O atoms in the equation. Add H+ ions when needed to balance H atoms in the equation

Example 1: A permanganate ion (Mn. O 4 -) and a thiosulfate ion (S 2 O 3 -2) react in an aqueous acidic solution to produce a sulfate ion and manganese (II) ion.

Example 3: Balance the following reaction occurring in an acidic solution. Cr 2 O 7 -2 + Cl- Cr+3 + Cl 2

Guidelines 1) Write down overall equation. 2) Determine oxidation and reduction half-reactions. 3) Balance the atoms. Add H 2 O to balance oxygens. Add H+ ions to balance hydrogens. 4) Balance the charges. Add electrons here. 5) Balance the half-reactions based on electron number need to have the same amount for oxidation and reduction. 6) Add the half-reactions and simplify.

Balancing Redox Reactions Basic Solutions

How do we recognize redox reactions occurring in basic solutions? Hydroxide ions (OH-) present instead of H+ ions How do we balance this redox reaction? ? Treat equation like a reaction occurring in an acidic solution Add hydroxide ions (OH-) to equal the H+ amount for the ENTIRE equation. Add OH- ions to BOTH sides ! ! ! If equal number of OH- and H+ ions on one side, combine into H 2 O molecules

Example 1: A disproportion occurs with Br 2 in a basic solution. Hint: balance redox equation normally until “Step 6: simplifying the overall equation” What in the world is a disproportion? Disproportion reaction-- A type of redox reaction where the same chemical substance is both oxidized and reduced.
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Activity formula
Activity formula Different types of redox reactions
Different types of redox reactions Types of reaction
Types of reaction 4 types of chemical reactions
4 types of chemical reactions What are the 5 types of chemical reactions
What are the 5 types of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions What are five chemical changes
What are five chemical changes 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Chapter 11 chemical reactions answers
Chapter 11 chemical reactions answers Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Three types of chemical reactions
Three types of chemical reactions Combustion reaction
Combustion reaction Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry 10 examples of redox reaction
10 examples of redox reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Stoichiometry island diagram
Stoichiometry island diagram I intro
I intro Predicting products of chemical reactions
Predicting products of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions
Chapter 10 chemical reactions The calculation of quantities in chemical reactions
The calculation of quantities in chemical reactions Principles of immuno chemical reactions
Principles of immuno chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting synthesis reactions
Predicting synthesis reactions Combination reaction equation
Combination reaction equation Unit 11 chemical reactions
Unit 11 chemical reactions Lesson 68 toxic reactions chemical equations
Lesson 68 toxic reactions chemical equations Www.biology-roots.com
Www.biology-roots.com Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life Reactants and products
Reactants and products Chemical reactions study guide
Chemical reactions study guide Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Equilibrium of chemical reactions
Equilibrium of chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review 5 chemical reactions
5 chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. Site:slidetodoc.com
Site:slidetodoc.com Chemical reactions summary
Chemical reactions summary