Chemical Reaction Energy All chemical reactions involve an






- Slides: 15

Chemical Reaction Energy All chemical reactions involve an energy change. The transfer of energy, usually heat, into or out of the reaction mixture. For example: q q When petroleum burns, heat is given out. When ammonium nitrate dissolves in water, heat is taken in.

Instead of writing ‘enthalpy change’ all the time, chemists use the symbols: ΔH Pronounced ‘delta H’. Δ = greek letter ‘delta’ meaning change. H = enthalpy (see below) So, ΔH means ‘change in enthalpy’. (Enthalpy is the heat content of a system; assuming pressure is constant)

Temperature Changes 1 Some reactions cause the temperature of the reaction mixture to increase. This type of reaction is called exothermic. Heat energy is given out by the reaction so the reaction feels hot.

Temperature Changes 2 Some reactions cause the temperature of the reaction mixture to decrease. This type of reaction is called endothermic. Heat energy is taken in by the reaction so the reaction feels cold.

Representing Energy Changes: The energy changes in a chemical reaction can be conveniently represented using energy level diagrams Energy level diagrams make it easier to decide whether a reaction is exothermic or endothermic See next slide for some examples.

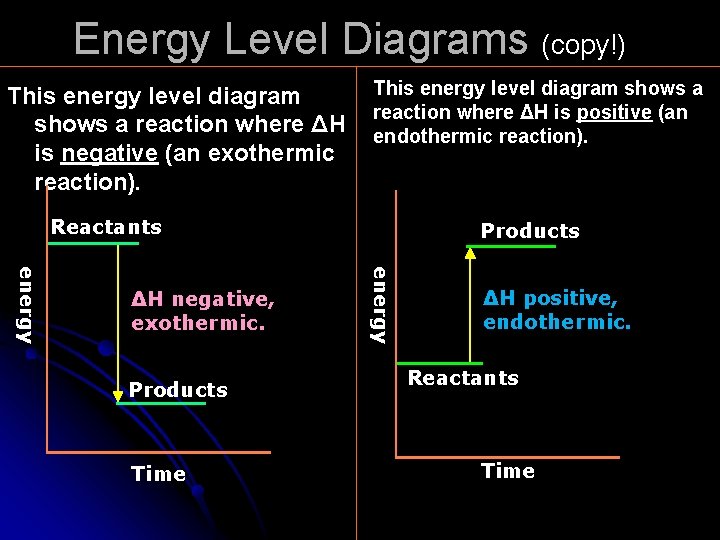
Energy Level Diagrams (copy!) This energy level diagram shows a reaction where ΔH is negative (an exothermic reaction). This energy level diagram shows a reaction where ΔH is positive (an endothermic reaction). Reactants Products Time energy ΔH negative, exothermic. Products ΔH positive, endothermic. Reactants Time

Making and Breaking Bonds: Breaking chemical bonds is always endothermic. Making chemical bonds is always exothermic. All chemical reactions involve bond ‘rearrangements’. Bonds are broken and new ones are formed. Chemical reactions are in two stages: 1. Breaking bonds, an endothermic process. 2. Making new bonds, an exothermic process.

Calculating Energy Changes: The energy change for a reaction can be calculated using ‘bond energies’. ‘Bond energy’ is the amount of energy required to break a bond. This value is always endothermic, so ΔH has a positive value. When a bond is formed the energy given out is exactly equal to, but of opposite sign, to the energy required to break the bond. Bond making is always an exothermic process; so ΔH has a negative value.

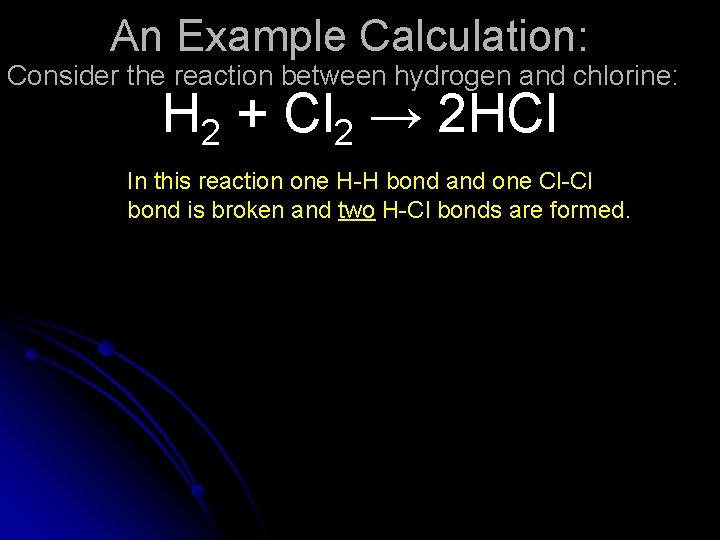
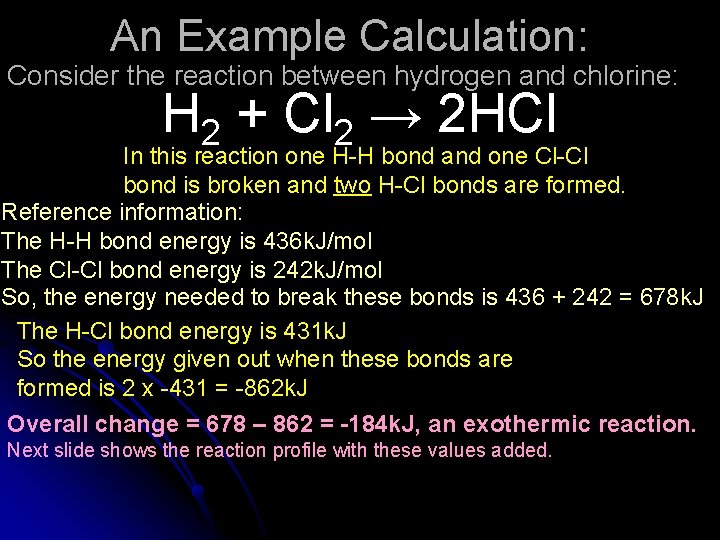
An Example Calculation: Consider the reaction between hydrogen and chlorine: H 2 + Cl 2 → 2 HCl In this reaction one H-H bond and one Cl-Cl bond is broken and two H-Cl bonds are formed.

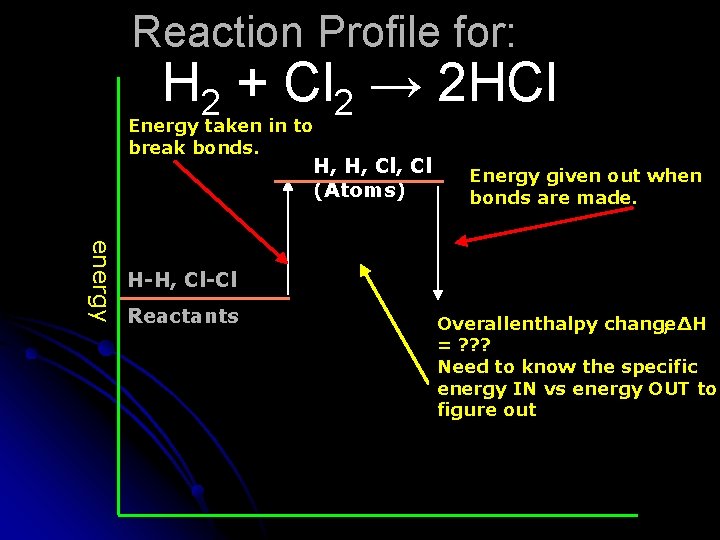
Reaction Profile for: H 2 + Cl 2 → 2 HCl Energy taken in to break bonds. H, H, Cl (Atoms) Energy given out when bonds are made. energy H-H, Cl-Cl Reactants Overall enthalpy change , ΔH = ? ? ? Need to know the specific energy IN vs energy OUT to figure out

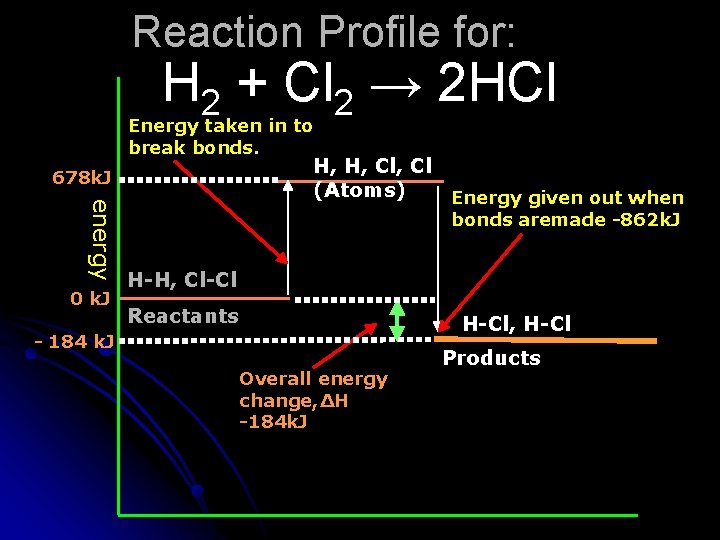
An Example Calculation: Consider the reaction between hydrogen and chlorine: H 2 + Cl 2 → 2 HCl In this reaction one H-H bond and one Cl-Cl bond is broken and two H-Cl bonds are formed. Reference information: The H-H bond energy is 436 k. J/mol The Cl-Cl bond energy is 242 k. J/mol So, the energy needed to break these bonds is 436 + 242 = 678 k. J The H-Cl bond energy is 431 k. J So the energy given out when these bonds are formed is 2 x -431 = -862 k. J Overall change = 678 – 862 = -184 k. J, an exothermic reaction. Next slide shows the reaction profile with these values added.

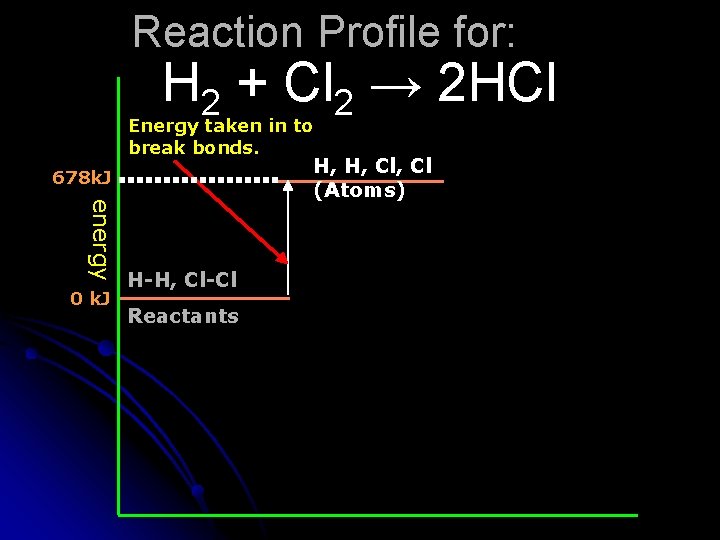
Reaction Profile for: H 2 + Cl 2 → 2 HCl Energy taken in to break bonds. 678 k. J energy 0 k. J H-H, Cl-Cl Reactants H, H, Cl (Atoms)

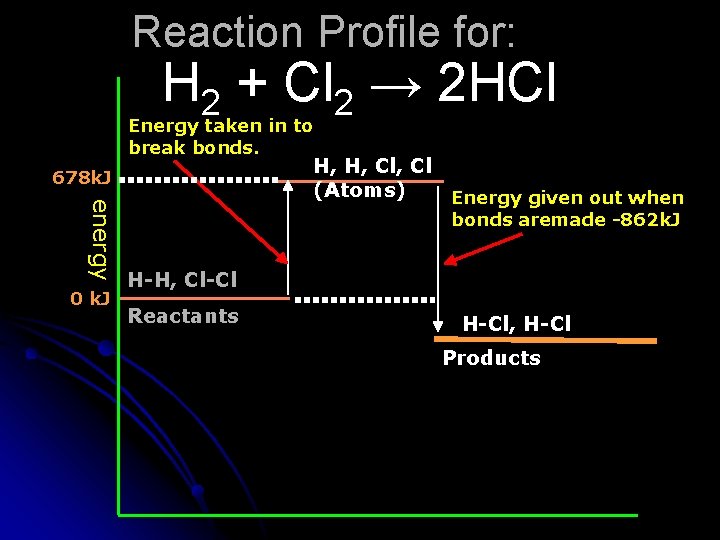
Reaction Profile for: H 2 + Cl 2 → 2 HCl Energy taken in to break bonds. 678 k. J energy 0 k. J H, H, Cl (Atoms) Energy given out when bonds aremade -862 k. J H-H, Cl-Cl Reactants H-Cl, H-Cl Products

Reaction Profile for: H 2 + Cl 2 → 2 HCl Energy taken in to break bonds. 678 k. J energy 0 k. J H, H, Cl (Atoms) Energy given out when bonds aremade -862 k. J H-H, Cl-Cl Reactants H-Cl, H-Cl - 184 k. J Overall energy change, ΔH -184 k. J Products

Summary Chemical reactions happen in two stages: 1. Breaking bonds, an endothermic process. 2. Making new bonds, an exothermic process. Exothermic rxns get hot, they give out heat. Endothermic rxns get cold, they take in heat. (think about that for a second) The energy changes in a chemical reaction can be represented using energy level diagrams and reaction profile diagrams.