Chemical Quantities Chapter 10 I The Mole Molar






















- Slides: 30

Chemical Quantities: Chapter 10 I. The Mole & Molar Conversions I II IV

A mole of paperclips Measure the length of a paper clip to the nearest 0. 1 cm. n If a mole is 6. 02 x 1023 items, how far will a mole of paper clips, placed end to end lengthwise, reach into space? n

How many light years (ly) would the paper clips extend into space? (1 ly = 9. 46 x 1015 m). n How does the distance you calculated compare with the following astronomical distances: nearest star (other than the sun) = 4. 3 ly, center of our galaxy = 30, 000 ly, nearest galaxy = 2 x 106 ly? n

A. What is the Mole? n A counting number (like a dozen) n Avogadro’s number (NA) n 1 mol = 6. 02 x 1023 items (atoms, molecules, formula units) n 1 mol is the SI unit used to measure amount of a substance A large amount!!!!

A. What is the Mole? n 1 mole of hockey pucks would equal the mass of the moon! n 1 mole of basketballs would fill a bag the size of the earth! n 1 mole of pennies would cover the Earth 1/4 mile deep!

B. Molar Mass n Mass of 1 mole of an element or compound. n Atomic mass tells the. . . · atomic mass units per atom (amu) · grams per mole (g/mol) n Round to 2 decimal places

B. Molar Mass Examples n carbon 12. 01 g/mol n aluminum 26. 98 g/mol n zinc 65. 39 g/mol

B. Molar Mass Examples n water · H 2 O · 2(1. 01) + 16. 00 = 18. 02 g/mol n sodium chloride · Na. Cl · 22. 99 + 35. 45 = 58. 44 g/mol

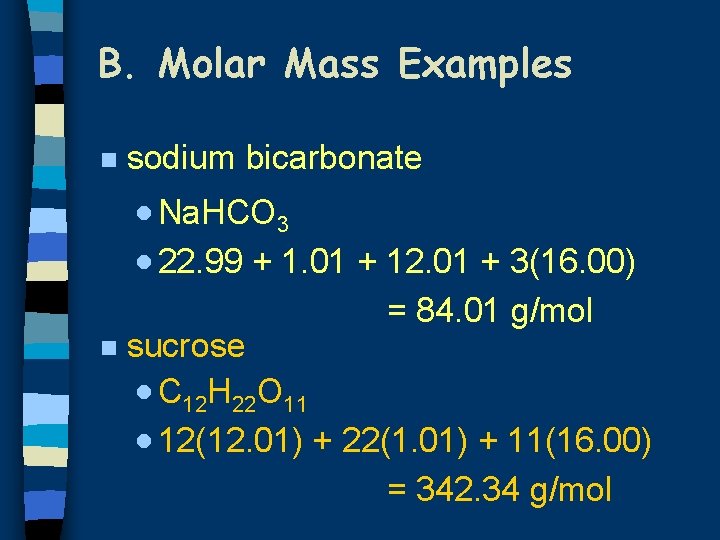
B. Molar Mass Examples n sodium bicarbonate · Na. HCO 3 · 22. 99 + 1. 01 + 12. 01 + 3(16. 00) = 84. 01 g/mol n sucrose · C 12 H 22 O 11 · 12(12. 01) + 22(1. 01) + 11(16. 00) = 342. 34 g/mol

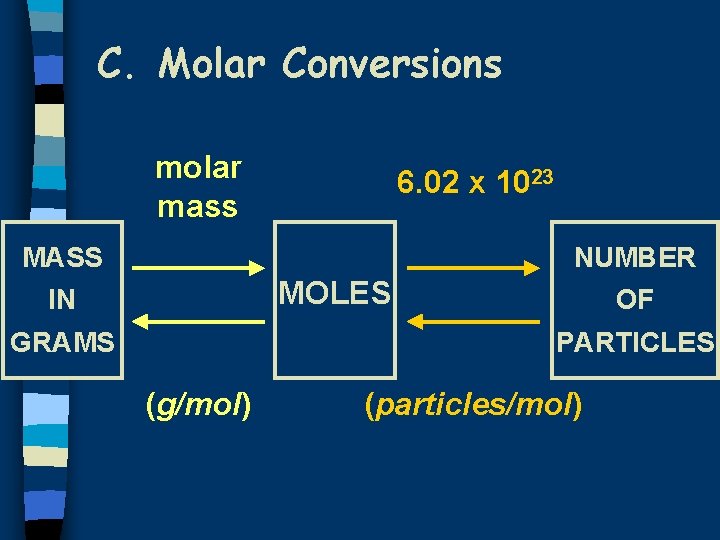
C. Molar Conversions molar mass 6. 02 x 1023 MASS NUMBER MOLES IN GRAMS OF PARTICLES (g/mol) (particles/mol)

C. Mole-Mass Conversions n Your class bought jellybeans in bulk to sell by the dozen. It’s too much work to count out each dozen, so you decide to measure the jellybeans by mass. You find that 1 dozen jellybeans equals 35 g. What mass of jellybeans should you measure if a customer wants 5 dozen jellybeans?

Jellybean Problem

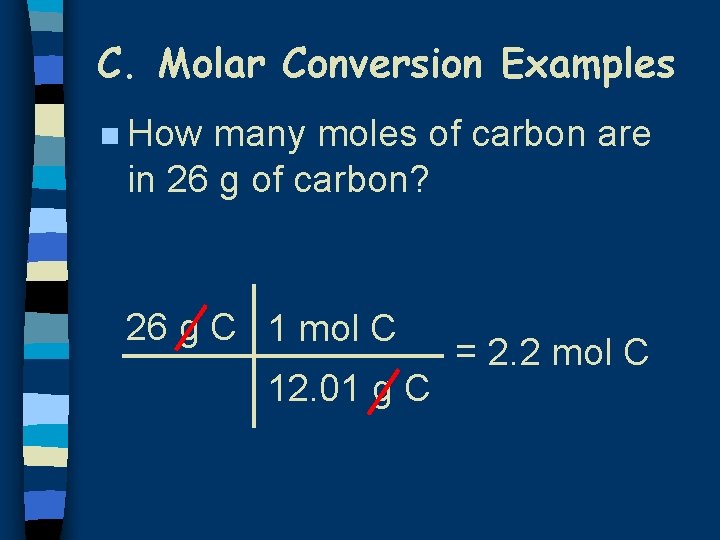
C. Molar Conversion Examples n How many moles of carbon are in 26 g of carbon? 26 g C 1 mol C 12. 01 g C = 2. 2 mol C

C. Molar Conversion Examples n You need 3. 00 mol of manganese for a chemical reaction, how can you measure that amount?

C. Mole-Particles Conversions n You buy 3. 5 dozen roses for your mom, how many roses did you buy?

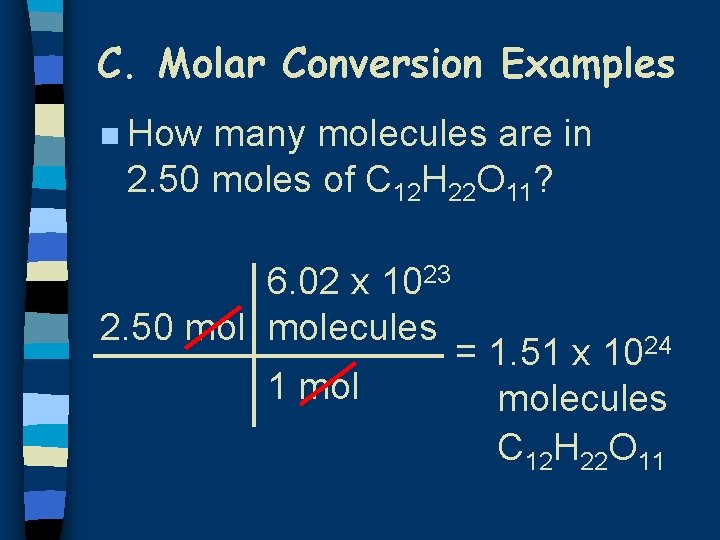
C. Molar Conversion Examples n How many molecules are in 2. 50 moles of C 12 H 22 O 11? 6. 02 x 1023 2. 50 molecules 1 mol = 1. 51 x 1024 molecules C 12 H 22 O 11

C. Molar Conversion Examples n Determine the number of atoms in 2. 50 mol of Zn.

C. Molar Conversion Examples n Calculate the number of moles that contain 3. 58 x 1023 formula units of Zn. Cl 2?

C. Mass-Moles- Particles Conversions n If 550 grams of jellybeans are unsold from your class fundraiser, without counting can you determine how many jellybeans this is? (remember you determined that 1 dozen jellybeans = 35 g)

Jellybean Problem 2

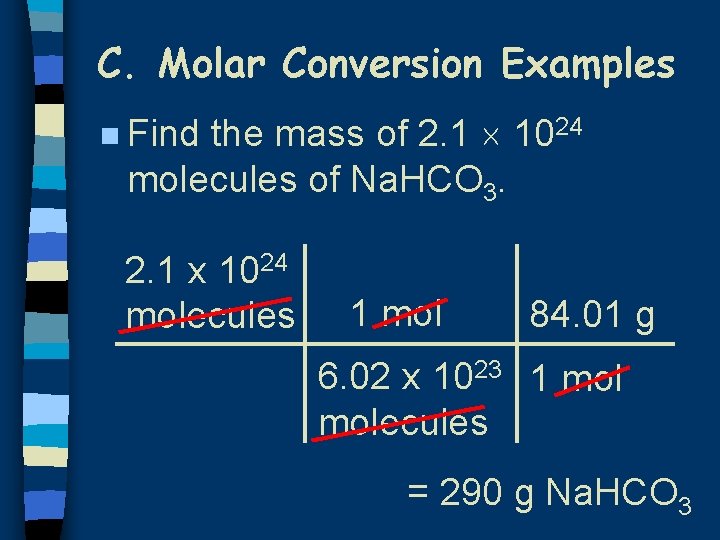
C. Molar Conversion Examples the mass of 2. 1 1024 molecules of Na. HCO 3. n Find 2. 1 x 1024 molecules 1 mol 84. 01 g 6. 02 x 1023 1 molecules = 290 g Na. HCO 3

C. Molar Conversion Examples n How many atoms are in 0. 230 g of Pb? n What is the mass in grams 1. 50 x 1015 atoms N?

Practice Problems n How many marbles are in 0. 200 mol of marbles?

Practice Problems n How many moles of magnesium is 1. 25 x 1023 atoms of magnesium?

Practice Problems n How many atoms are in 2. 12 mol of propane (C 3 H 8)?

Practice Problems n How many moles are there in 4. 65 x 1024 molecules of NO 2?

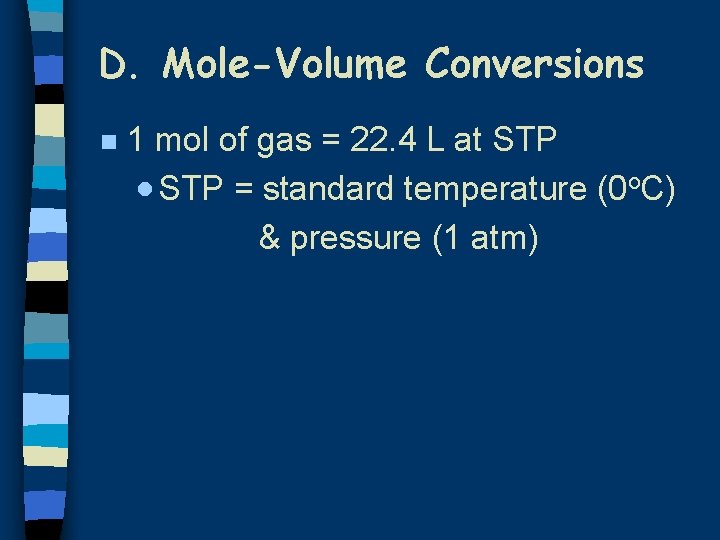
D. Mole-Volume Conversions n 1 mol of gas = 22. 4 L at STP · STP = standard temperature (0 o. C) & pressure (1 atm)

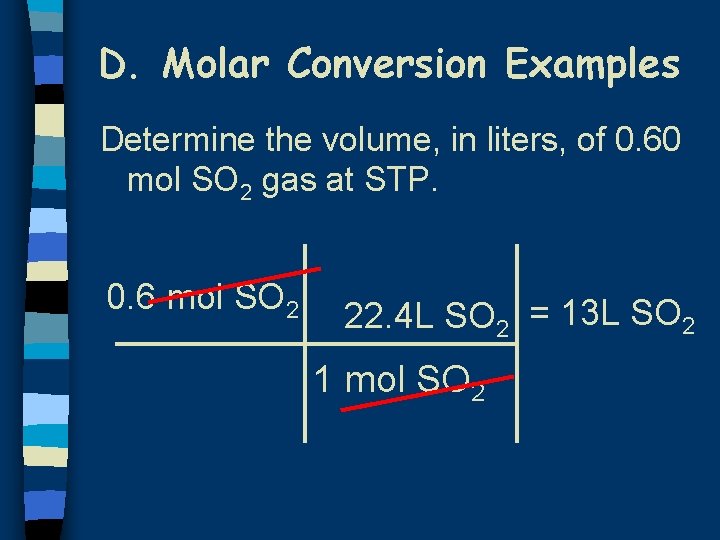
D. Molar Conversion Examples Determine the volume, in liters, of 0. 60 mol SO 2 gas at STP. 0. 6 mol SO 2 22. 4 L SO 2 = 13 L SO 2 1 mol SO 2

D. Molar Conversion Examples n Assuming STP, how many moles are in 0. 880 L He? 3. 93 x 10 -2 mol He n What is the volume at STP of 3. 70 mol N 2? 82. 9 L N 2

D. Molar Conversions The density of a gaseous compound containing carbon and oxygen is 1. 964 g/L at STP. Determine the molar mass of the compound. 44. 0 g/mol n What is the density of krypton gas at STP? 3. 74 g/L n