Chemical Proportions in Compounds Chapter 3 Chemical Composition

































- Slides: 45

Chemical Proportions in Compounds Chapter 3

Chemical Composition • The chemical make-up or composition of a compound can be described in 2 ways: – Formula – which will tell the ratio of each type of atom in the compound, ex. H 2 O – Percent composition by mass - which gives the percent by mass of each element in the compound.

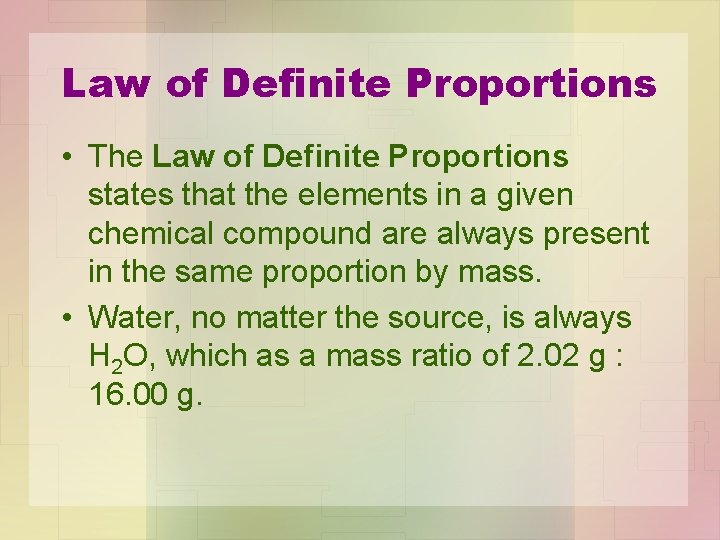
Law of Definite Proportions • The Law of Definite Proportions states that the elements in a given chemical compound are always present in the same proportion by mass. • Water, no matter the source, is always H 2 O, which as a mass ratio of 2. 02 g : 16. 00 g.

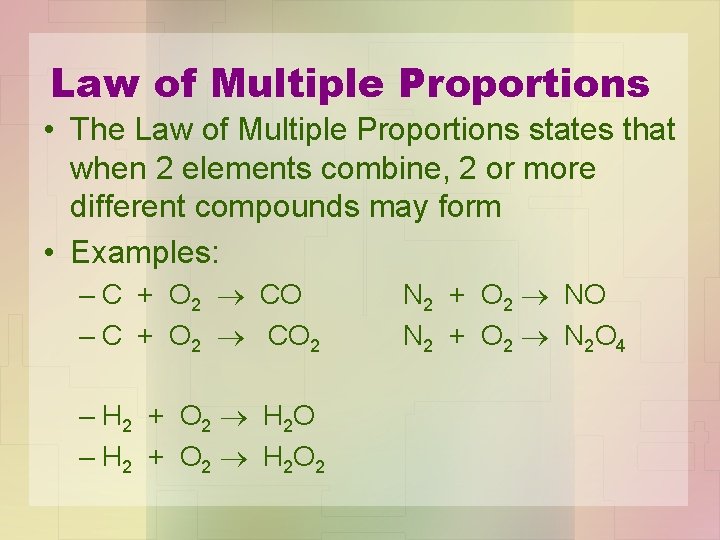
Law of Multiple Proportions • The Law of Multiple Proportions states that when 2 elements combine, 2 or more different compounds may form • Examples: – C + O 2 CO 2 – H 2 + O 2 H 2 O 2 N 2 + O 2 NO N 2 + O 2 N 2 O 4

• Each compound has its own unique properties. • What compound will form, would depend on the experimental conditions (temperature, pressure, catalysts, activation energy, etc. ).

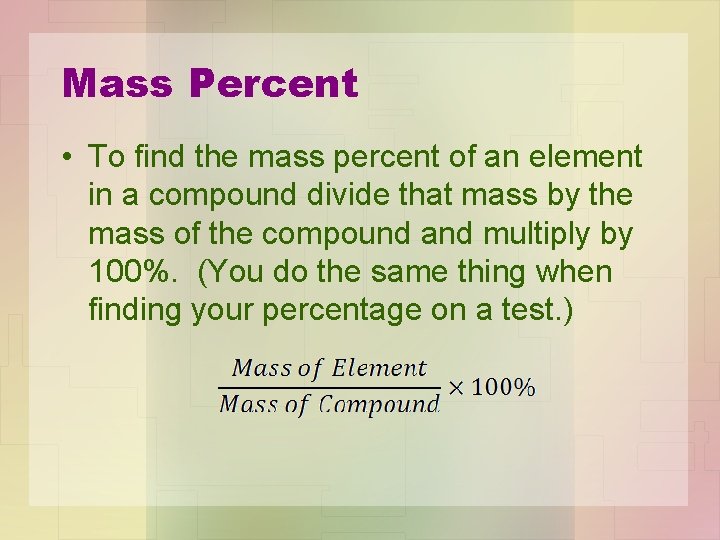
Mass Percent • To find the mass percent of an element in a compound divide that mass by the mass of the compound and multiply by 100%. (You do the same thing when finding your percentage on a test. )

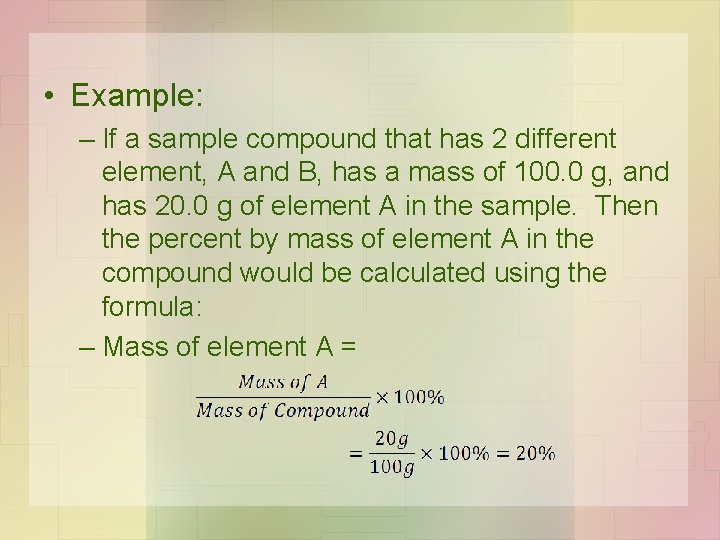
• Example: – If a sample compound that has 2 different element, A and B, has a mass of 100. 0 g, and has 20. 0 g of element A in the sample. Then the percent by mass of element A in the compound would be calculated using the formula: – Mass of element A =

• By subtraction you can calculate the percent of element B. – Element B = 100% - 20% = 80% Or just use the formula again solving for the mass of element B.

Percentage Composition • We have used the mole to describe the composition of compounds. • One mole of carbon dioxide contains 1 mole of carbon to 2 moles of oxygen. • The percentage composition of a compound refers to the relative mass of each element in the compound. • You need to find the mass percent of every element in the compound.

• Example: – Vanillin – C 8 H 8 O 3 contains 63. 1% carbon, 5. 3% hydrogen, and 31. 6% oxygen – Why don’t the subscripts and percentages seem to agree? – Remember it is based on mass. Hydrogen has by far the smallest mass. • Percentage composition can be found experimentally and used to help identify unknown compounds.

• Do Sample Problem on Page 81 • Do Practice Problems on Page 82 #s 14 • Do Thought Lab on Page 83

Calculating Percentage Composition from a Chemical Formula • In the previous practice problems you used mass data to find the percentage composition. • This method is used to identify unknown substances in a lab. • Another use of percentage composition is finding the exact amount of an element in a known substance for extracting purposes.

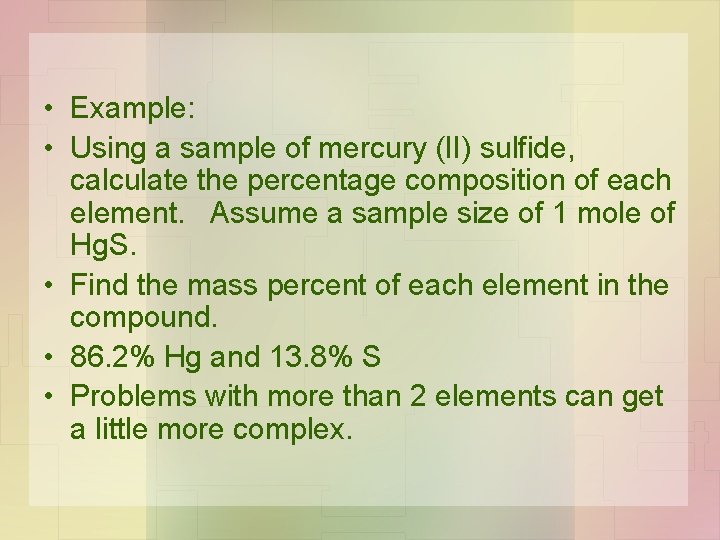
• Example – mercury is often found in nature as mercury (II) sulfide. • Knowing the percentage composition of Hg. S, enables a metallurgist to predict the amount of mercury that can be extracted.

• The sample size is not important to determine the percentage composition by mass of a homogeneous sample. • According to the law of definite proportions, there is a fixed proportion of each element in the compound, no matter the size. • This means you can chose a convenient sample size.

• Example: • Using a sample of mercury (II) sulfide, calculate the percentage composition of each element. Assume a sample size of 1 mole of Hg. S. • Find the mass percent of each element in the compound. • 86. 2% Hg and 13. 8% S • Problems with more than 2 elements can get a little more complex.

• Do Sample Problem on Page 84 • Do Practice Problems on Page 85 #s 58 • Do Section Review on Page 86 #s 1 -6

The Empirical Formula of a Compound Section 3. 2 • Atoms combine with each other in simple whole number ratios. • The empirical formula is the simplest formula of the compound. It is the lowest whole number ratio. • The molecular formula is the actual formula. It says exactly how many atoms are in the compound.

• See Table 3. 1 on Page 87 • The molecular formula is a multiple of the empirical formula. • You can determine a compound’s empirical formula from its percentage composition.

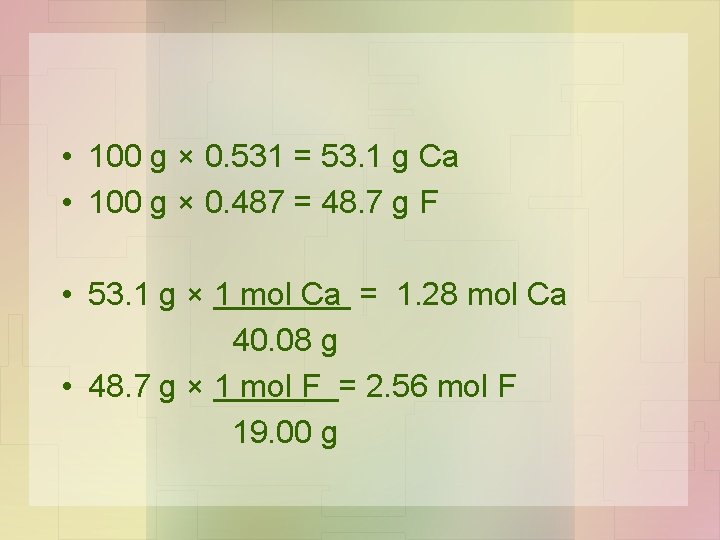
• If we know the mass percentages of the elements in the compound we can determine the simplest formula. • Example: Calcium and fluorine react to form a compound called fluorite. 1. Using the molar mass you can determine the percentage composition of fluorite. 51. 3% Ca and 48. 7% F

2. Always assume a 100 g sample (it makes the calculation to grams simpler). 3. Convert the grams to moles (use molar mass). 4. Look at the ratio of moles of each element that reacted to determine the mole ratio. (Divide the largest by the smallest. )

• 100 g × 0. 531 = 53. 1 g Ca • 100 g × 0. 487 = 48. 7 g F • 53. 1 g × 1 mol Ca = 1. 28 mol Ca 40. 08 g • 48. 7 g × 1 mol F = 2. 56 mol F 19. 00 g

• 2. 56 mol F = 2 1. 28 mol Ca 1 • Or 2 F: 1 Ca or Ca. F 2 • The simplest ratio of atoms or moles is 1 Ca for 2 F. Therefore the empirical formula for fluorite is Ca. F 2.

• Do Sample Problem on Page 88 • Do Practice Problems on Page 89 #s 912

Helpful Hints… • Do not round until the end • Use the maximum number of digits you can. • Round close numbers to the nearest whole number. 0. 95 to 0. 99 round up. 0. 01 to 0. 04 round down. 0. 45 to 0. 55 round to 0. 5. • By dividing by the smallest mole amount, one atom will always have the subscript 1.

• Not all compounds contain ratios that include 1 atom of something, e. x Fe 2 O 3. • You may need to multiply the ratios to get the numbers to turn into whole numbers. • See Table 3. 2 on Page 90

• Example: • If you get to the end of your calculations and end up with something like this… 2. 94 mol Y = 1. 5 mol Y × 2 = 3 mol Y 1. 96 mol X 1 mol X 2 2 mol X So the empirical formula would be X 2 Y 3

• Do Sample Problem on Page 90 • Do Practice Problems on Page 91 #s 13 -16 • Do Section Review on Page 94

The Molecular Formula of a Compound Section 3. 3 • Read Page 95

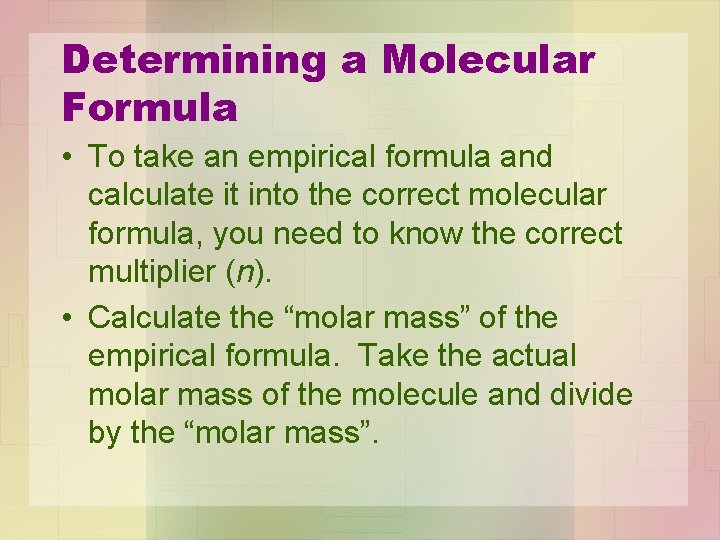
Determining a Molecular Formula • To take an empirical formula and calculate it into the correct molecular formula, you need to know the correct multiplier (n). • Calculate the “molar mass” of the empirical formula. Take the actual molar mass of the molecule and divide by the “molar mass”.

• This whole number ratio is the multiplier (n). • Multiply the EF by this number to obtain the MF. • The actual molar mass must be given.

• Do Sample Problem on Page 97 • Do Practice Problems on Page 97 #s 17 -19 • Do Section Review on Page 98

Finding Empirical and Molecular Formulas by Experiment • One method of finding the percentage composition and empirical formula is by taking a known mass of an unknown substance and reacting it with oxygen to find the mass of the product. • This works for simple compounds that react in predictable ways.

The Carbon-Hydrogen Combustion Analyzer • This process is used for chemicals that are mainly hydrogen, carbon, and oxygen. • A combustion reaction is the burning of hydrocarbons (CH) chains in the presence of oxygen to produce carbon dioxide and water.

• The carbon-hydrogen combustion analyzer burns compounds in a stream of pure oxygen to yield carbon dioxide and water. • If you find the mass of the carbon dioxide and water separately then you can determine the mass percent of carbon and hydrogen in the compound.

• How it works – see Figure 3. 7 on Page 99. • A sample is placed in a furnace and it is heated as pure oxygen is streamed in. • The products of the reaction leave the furnace and pass through filters. • The water vapor is collected by passing through a tube of magnesium perchlorate (Mg(Cl. O 4)2). .

• The Mg(Cl. O 4)2 absorbs all the water. • The difference in the mass of the tube, before and after the reaction, is the mass of the water. • All the hydrogen in the sample was converted to water. • Because hydrogen is always 11. 2% of water, you can find the amount of hydrogen from the sample.

• Take the percent composition and multiply by the mass of the water and you get the mass of the hydrogen that was reacted. • A second filter captures the carbon dioxide. The same process of multiplying the mass of CO 2 by the mass percent of carbon in CO 2 tells you how much carbon was in the sample.

• The carbon-hydrogen combustion analyzer can be used if the sample contains a third element. • Anything not accounted for by the hydrogen or carbon must be another element. • Finally, once you have the mass of each element you can find the empirical formula.

• Sample Problem on Page 100 • Practice Problems on Page 101 #s 21 and 22

Hydrated Ionic Compounds • Hydrates are compounds that have water molecules bonded to the formula unit. • The water molecules are actually part of the crystal lattice structure. • Hydrates have a specific number of water molecules. • Chemists usually know the ionic part but may have to determine the number of water molecules.

• Hydrates occur when ionic compounds crystallize from a water solution with water molecules incorporated into their crystal structure. • Some hydrates will liquefy when in contact with water vapor. • Hydrates are used as desiccants – absorb moisture from the air.

• Hydrates are written as an ionic compound weakly bonded to a number of water molecules: • Magnesium sulfate heptahydrate Mg. SO 4● 7 H 2 O • Every formula unit of Mg. SO 4 has seven water molecules weakly bonded to it. • The dot is used to write hydrate formulas and means weakly bonded to it and not as a multiplication sign.

• Several ionic compounds can also occur as hydrates. • The term anhydrous means not in hydrate form. • The molar mass of a hydrate includes all water molecules. • Therefore the mass of Mg. SO 4● 7 H 2 O is higher than that of the anhydrous Mg. SO 4.

• Calculations for percent mass and empirical formula for hydrates are similar to those already done. • Do Sample Problem on Page 102 • Do Practice Problems on Page 103 #s 23 and 24.

• Do Section Review on Page 106 #s 1 -3, 6, & 7 • Do Chapter 3 Review on Pages 107 109 #s 1 - 22
 Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Ionic covalent and metallic bonds
Ionic covalent and metallic bonds Ionic compound properties
Ionic compound properties Chapter 7 chapter assessment ionic compounds and metals
Chapter 7 chapter assessment ionic compounds and metals Chapter 19 confidence intervals for proportions
Chapter 19 confidence intervals for proportions Chapter 7 proportions and similarity answers
Chapter 7 proportions and similarity answers Chapter 22 comparing two proportions
Chapter 22 comparing two proportions Chapter 20 testing hypotheses about proportions
Chapter 20 testing hypotheses about proportions Chapter 18 confidence intervals for proportions
Chapter 18 confidence intervals for proportions Chapter 21 comparing two proportions
Chapter 21 comparing two proportions Chapter 19 testing hypotheses about proportions
Chapter 19 testing hypotheses about proportions Chapter 19 confidence intervals for proportions
Chapter 19 confidence intervals for proportions 7-2 properties of proportions answers
7-2 properties of proportions answers Chapter 12 inference for proportions answers
Chapter 12 inference for proportions answers Chapter 20 testing hypotheses about proportions
Chapter 20 testing hypotheses about proportions Chapter 22 comparing two proportions
Chapter 22 comparing two proportions Chapter 22 comparing two proportions
Chapter 22 comparing two proportions Chapter 22 comparing two proportions
Chapter 22 comparing two proportions Writing formulas criss-cross method
Writing formulas criss-cross method Writing names for ionic compounds
Writing names for ionic compounds Naming compunds
Naming compunds Unit chemical bonding forming ionic compounds ws 2
Unit chemical bonding forming ionic compounds ws 2 Deca silicon trinitride formula
Deca silicon trinitride formula Chemical formula vs molecular formula
Chemical formula vs molecular formula Hcn binary or ternary
Hcn binary or ternary Naming chemical compounds flowchart
Naming chemical compounds flowchart Chemical compounds
Chemical compounds Are kc and kp equal
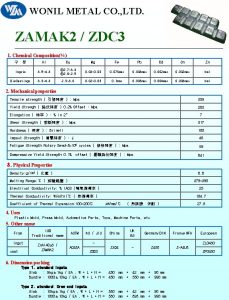
Are kc and kp equal Zamak 5 chemical composition
Zamak 5 chemical composition Mining company profile
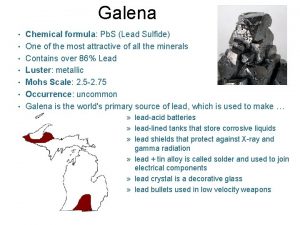
Mining company profile Chemical composition
Chemical composition Sodium abbreviation periodic table
Sodium abbreviation periodic table Limonite molecular formula
Limonite molecular formula Composition of fungi
Composition of fungi What is the chemical composition of saliva
What is the chemical composition of saliva Zamak 5 composition
Zamak 5 composition Mesosphere chemical composition
Mesosphere chemical composition Chemical composition of solid waste
Chemical composition of solid waste Urea reactor design
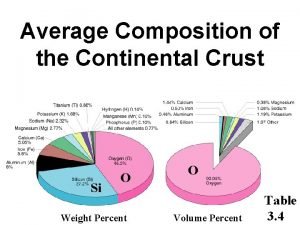
Urea reactor design Chemical composition of continental crust
Chemical composition of continental crust Zamak 3 chemical composition
Zamak 3 chemical composition Chemical composition in a cell
Chemical composition in a cell P
P Chemical composition of gene
Chemical composition of gene Filament bending
Filament bending