Chemical Process Industries Chemical Engineering Department 1 CHPE




















- Slides: 52

Chemical Process Industries Chemical Engineering Department 1

謝志誠 CHPE 306 Chemical Process Industries • Spring 2015 -2016 • Monday, Wednesday 12: 00 - 1: 15 PM • Instructor : Marilyn M. Bojangin • Room 16 L

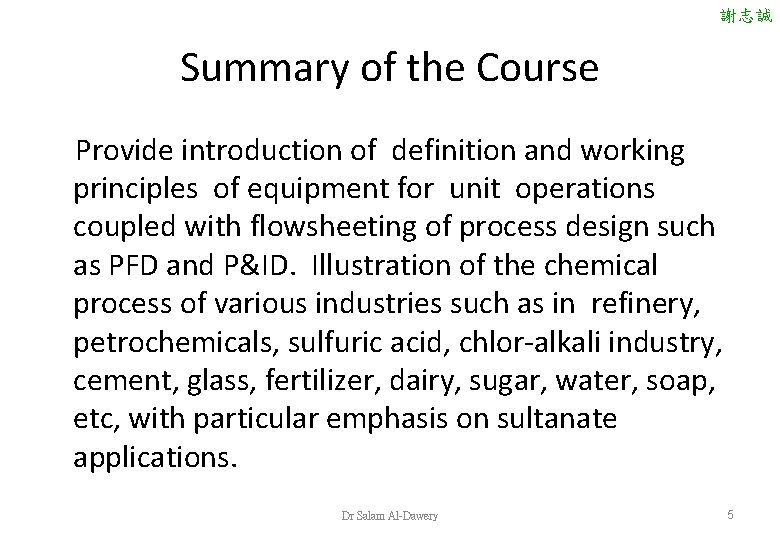
Chemical Process Industries ASSESSMENTS Course Works Assignments (2) 10% Quizzes (2 ) 10% Project and /or Site visit Reports (2) Two In-term exams (2) Final Examination 10% 30% 40% REFERENCES Austin, G. T. , "Shreve's Chemical Process Industries", 5 thed Mc. Graw- Hill: New York, 1984. 謝志誠

謝志誠 Introduction

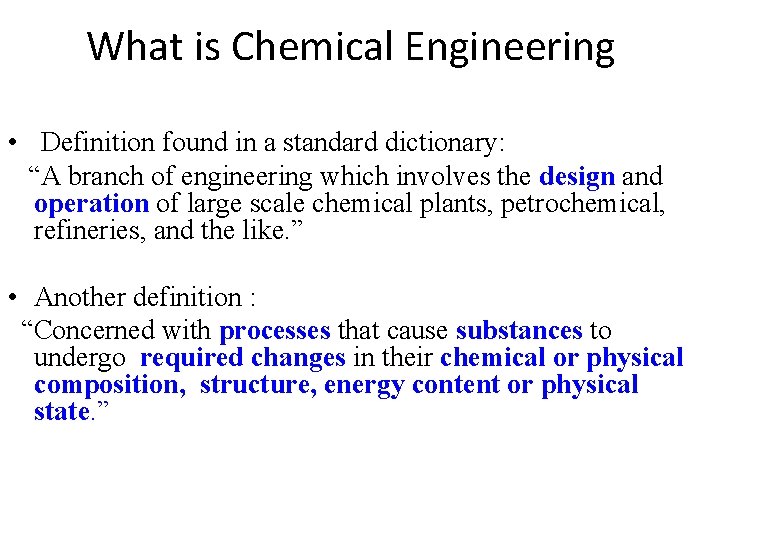
謝志誠 Summary of the Course Provide introduction of definition and working principles of equipment for unit operations coupled with flowsheeting of process design such as PFD and P&ID. Illustration of the chemical process of various industries such as in refinery, petrochemicals, sulfuric acid, chlor-alkali industry, cement, glass, fertilizer, dairy, sugar, water, soap, etc, with particular emphasis on sultanate applications. Dr Salam Al-Dawery 5

謝志誠 Objective of the Course – Identify the relationship between industrial chemistry and chemical engineering. – Explain the basic operation of the chemical industry. – Define a process flow diagram (PFD) and a process and instrumentation diagram (P&ID). – Create a PFD from a written description of a process. – Evaluate feeds and products from written descriptions of chemical processes. – Conduct actual survey of the process operation by visiting the plants. – Practice research a topic independently or as a team member, prepare a written and report, and make an oral presentation Dr Salam Al-Dawery 6

Chapter One Definition of Chemical Engineering & Unit Operations

What is Chemical Engineering • Definition found in a standard dictionary: “A branch of engineering which involves the design and operation of large scale chemical plants, petrochemical, refineries, and the like. ” • Another definition : “Concerned with processes that cause substances to undergo required changes in their chemical or physical composition, structure, energy content or physical state. ”

Chemical Engineer Tasks • Chemical Engineers convert scientific discoveries into marketable products. • They are involved in many aspects of chemical production, research, and design, as well as in the construction and operation of industrial plants. • They design equipment for safe storage and transportation of chemical solids, liquids, and gases, • Design control systems for chemical plants based upon data from lab experiments and pilot plant operations. • Chemical Engineers also perform tests and take measurements in order to determine the most efficient production methods

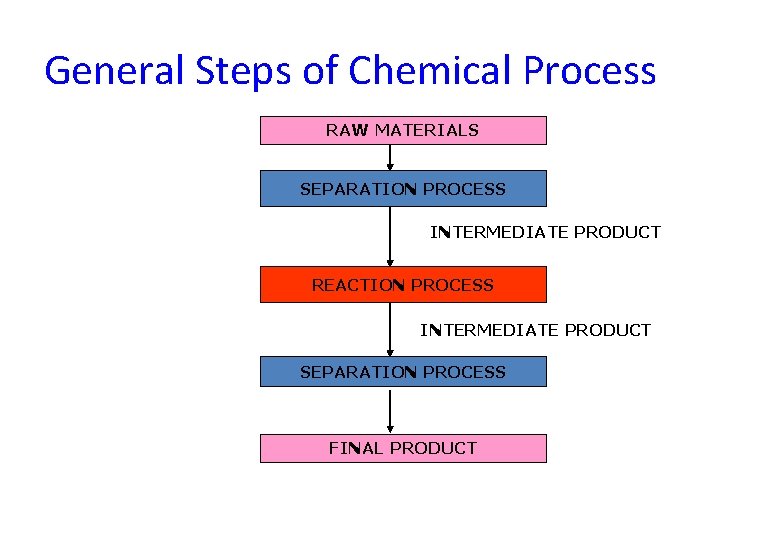
General Steps of Chemical Process RAW MATERIALS SEPARATION PROCESS INTERMEDIATE PRODUCT REACTION PROCESS INTERMEDIATE PRODUCT SEPARATION PROCESS FINAL PRODUCT

Chemical Process Design • There is no standard steps • There is no single correct solution • There is always a need to find a better solution from several alternatives

Given Information for a Process Design • • Products needed and production rate Purity of the desired product Raw material to be used Utility available The process route Expected market Site selection

Results of Process Design Project • • • Process flowsheet description Mass and energy balances results Equipment sizing and specification Economic feasibility analysis Environmental requirements The final report

Plant Operation • In the design of an industrial plant, the methods which will be used for plant operation and control help to determine many of the design variables. For example, the extent of instrumentation can be a factor in choosing the type of process and setting the labor requirements. • It should be remembered that maintenance work will be necessary to keep the installed equipment and facilities in good operating condition.

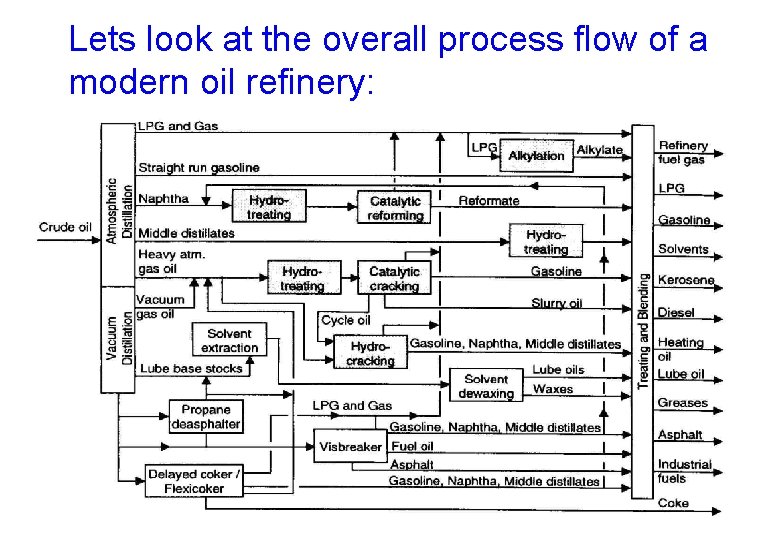
Lets look at the overall process flow of a modern oil refinery:

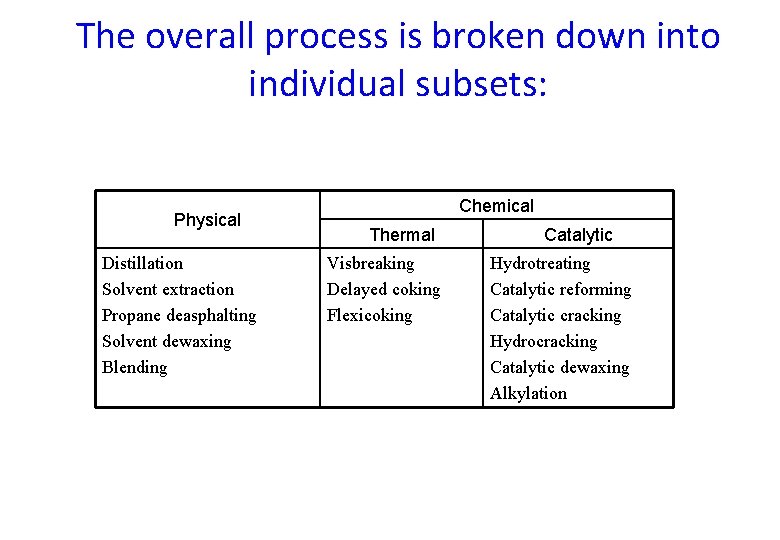
The overall process is broken down into individual subsets: Physical Distillation Solvent extraction Propane deasphalting Solvent dewaxing Blending Chemical Thermal Visbreaking Delayed coking Flexicoking Catalytic Hydrotreating Catalytic reforming Catalytic cracking Hydrocracking Catalytic dewaxing Alkylation

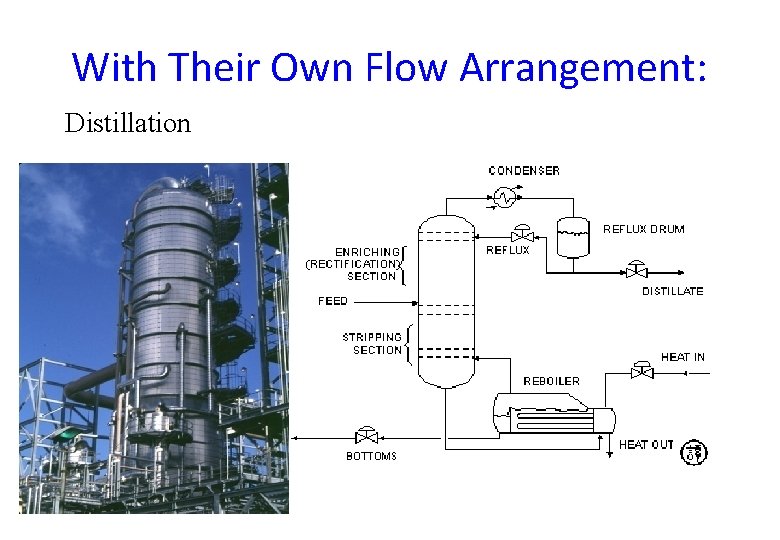
With Their Own Flow Arrangement: Distillation

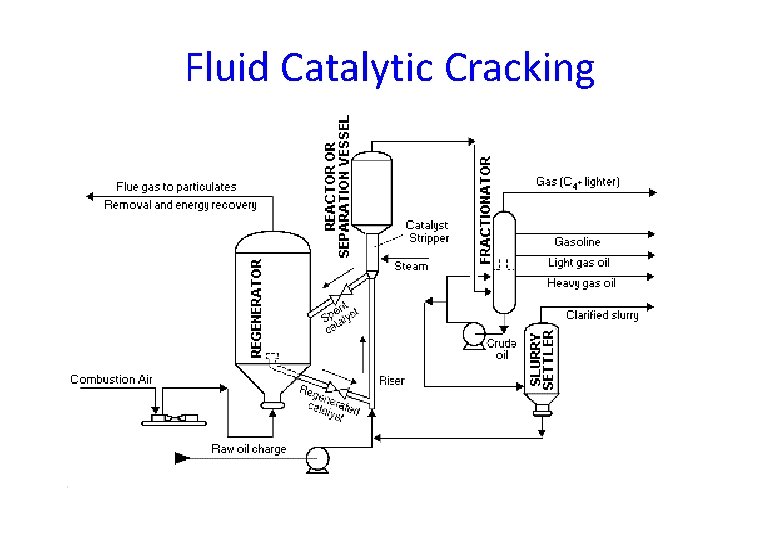
Fluid Catalytic Cracking

We notice…. . • The number of individual processes is large, each one can be broken down into a series of steps that appear in process after process • The individual “steps” have common techniques and are based upon the same scientific principles Fluid Dynamics Heat Transfer Evaporation Humidification Gas absorption Solvent Extraction Adsorption Distillation Drying Mixing Classification Fluidization Filtration Screening Crystallization Centrifugation Materials handling

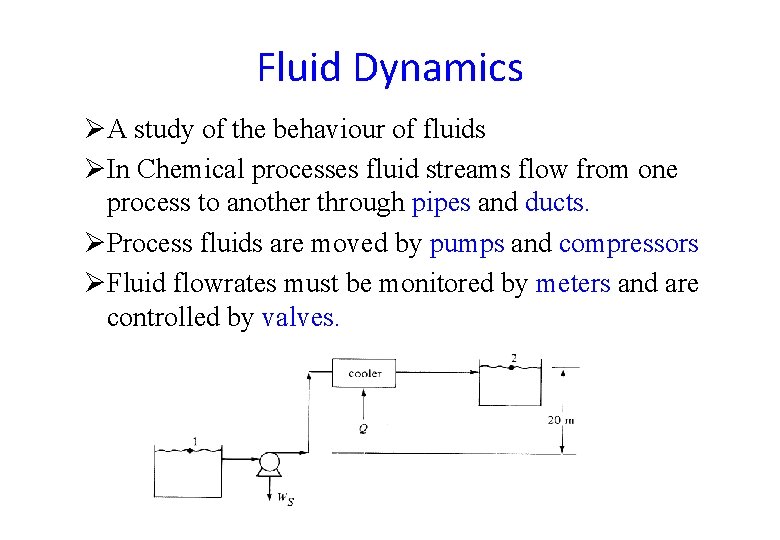
Fluid Dynamics ØA study of the behaviour of fluids ØIn Chemical processes fluid streams flow from one process to another through pipes and ducts. ØProcess fluids are moved by pumps and compressors ØFluid flowrates must be monitored by meters and are controlled by valves.

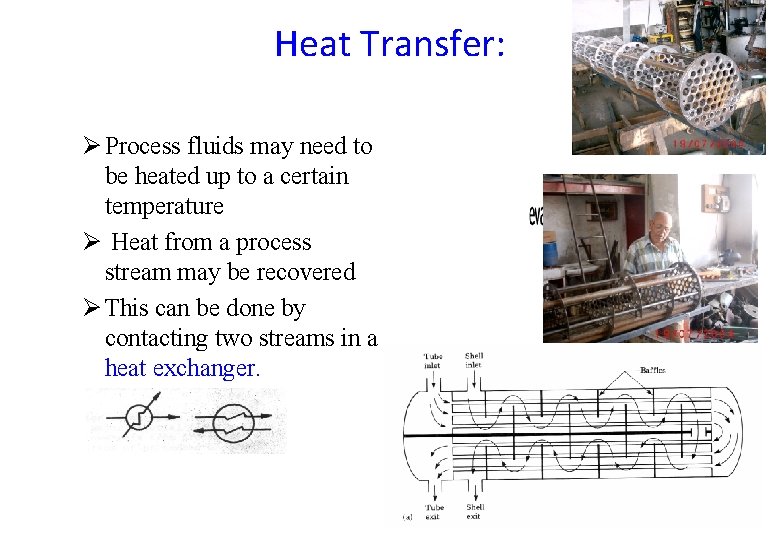
Heat Transfer: Ø Process fluids may need to be heated up to a certain temperature Ø Heat from a process stream may be recovered Ø This can be done by contacting two streams in a heat exchanger.

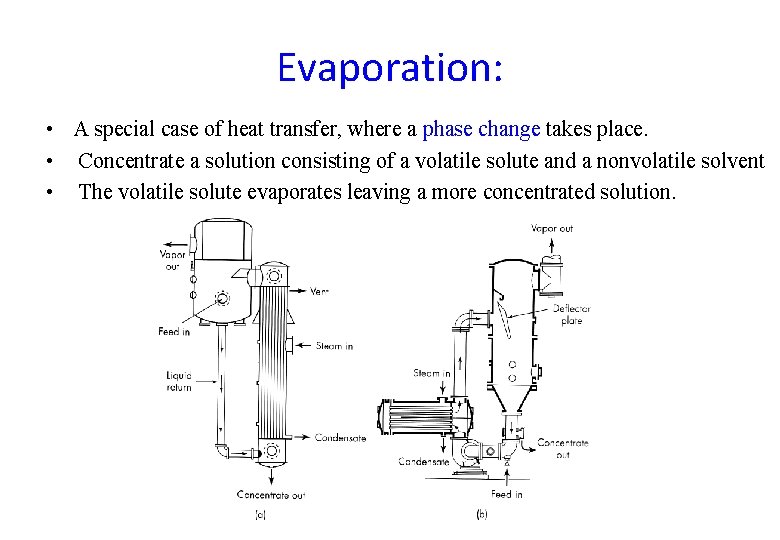
Evaporation: • A special case of heat transfer, where a phase change takes place. • Concentrate a solution consisting of a volatile solute and a nonvolatile solvent • The volatile solute evaporates leaving a more concentrated solution.

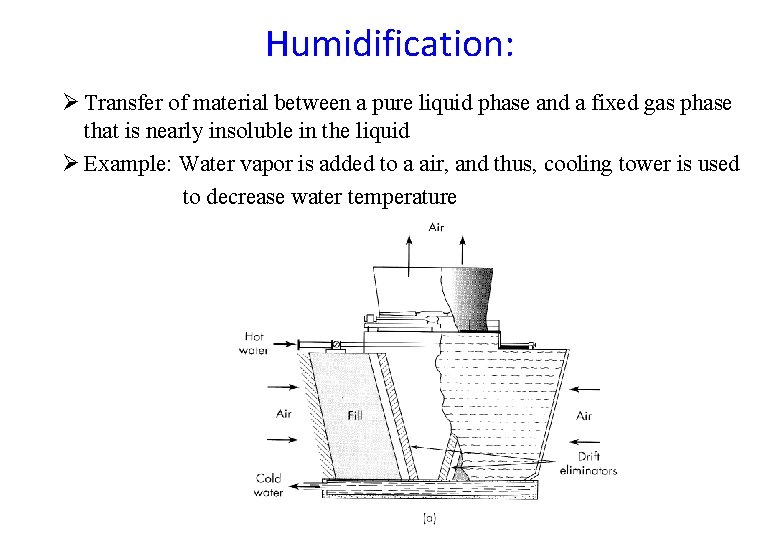
Humidification: Ø Transfer of material between a pure liquid phase and a fixed gas phase that is nearly insoluble in the liquid Ø Example: Water vapor is added to a air, and thus, cooling tower is used to decrease water temperature

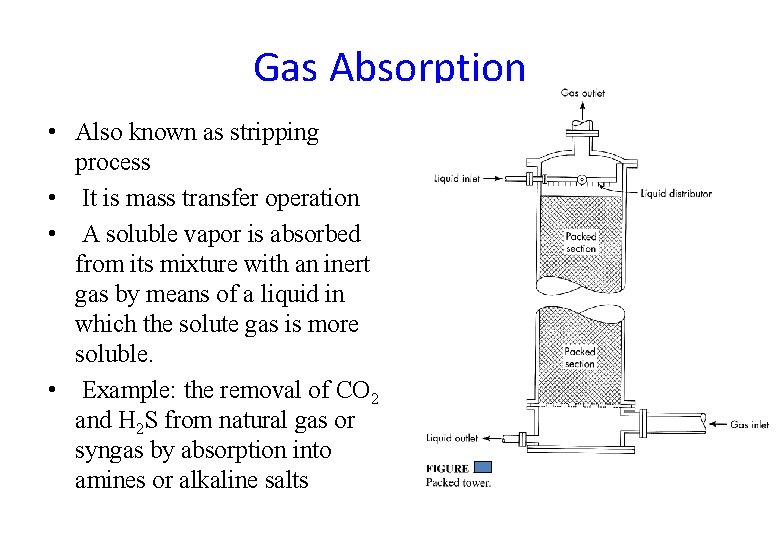
Gas Absorption • Also known as stripping process • It is mass transfer operation • A soluble vapor is absorbed from its mixture with an inert gas by means of a liquid in which the solute gas is more soluble. • Example: the removal of CO 2 and H 2 S from natural gas or syngas by absorption into amines or alkaline salts

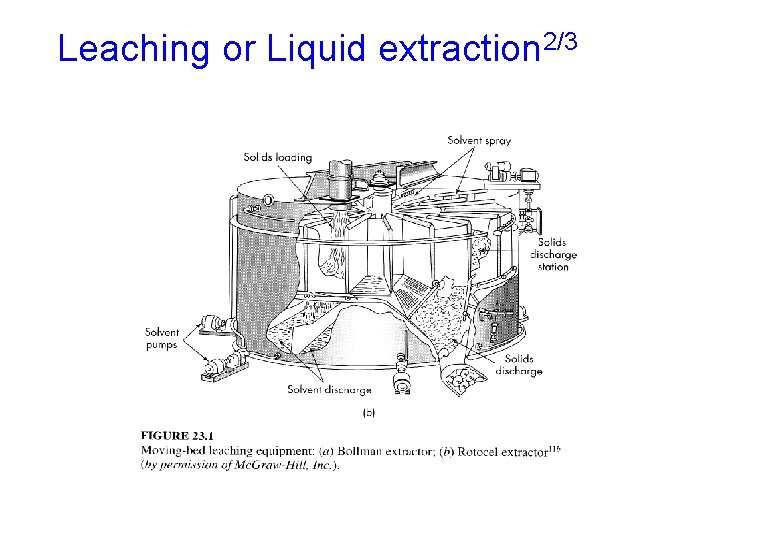
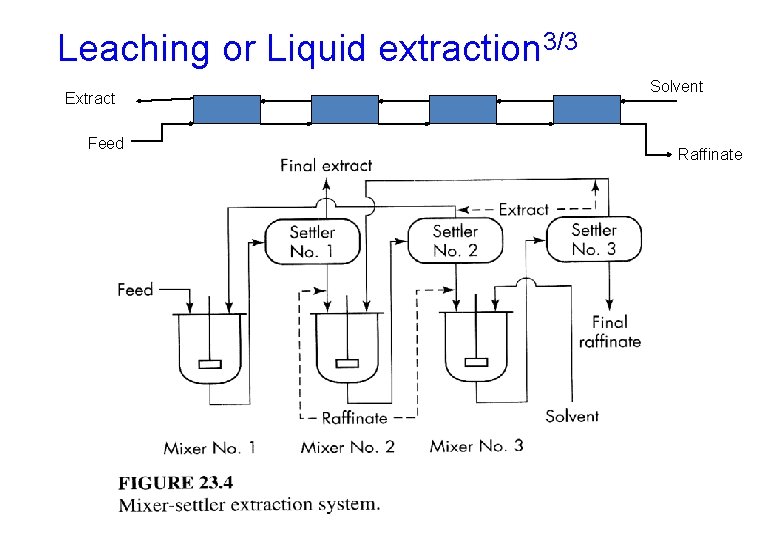
Leaching or Liquid extraction 1/3 • Leaching is a process in which the solid extraction involves the dissolving of soluble matter from its mixture with an insoluble solid • Liquid extraction is the separation of two miscible liquids by the use of a solvent that preferentially dissolves one of them. • Liquid extraction an alternative to distillation for difficult separations • Example: leaching oil from seeds, and penicillin is separated from fermentation broth by extraction with butyl acetate

Leaching or Liquid extraction 2/3

Leaching or Liquid extraction 3/3 Extract Feed Solvent Raffinate

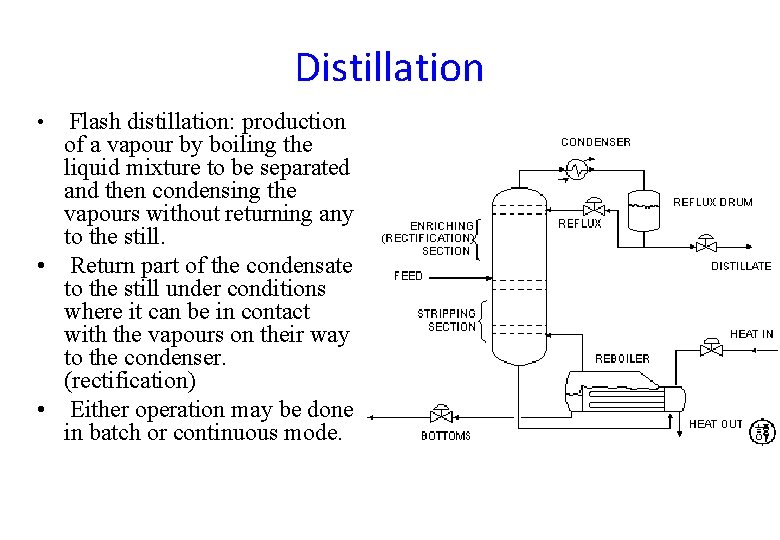
Distillation Flash distillation: production of a vapour by boiling the liquid mixture to be separated and then condensing the vapours without returning any to the still. • Return part of the condensate to the still under conditions where it can be in contact with the vapours on their way to the condenser. (rectification) • Either operation may be done in batch or continuous mode. •

How does distillation work? 1/3 • Distillation is defined as: Øa process in which a liquid or vapour mixture of two or more substances is separated into its component fractions of desired purity, by the application and removal of heat.

How does distillation work? 2/3 • Distillation is based on the fact that the vapour of a boiling mixture will be richer in the components that have lower boiling points. • Thus, when this vapour is cooled and condensed, the condensate will contain the more volatile components. At the same time, the original mixture will contain more of the less volatile components. • Distillation is the most common separation technique and it consumes enormous amounts of energy, both in terms of cooling and heating requirements. • Distillation can contribute to more than 50% of plant operating costs.

How does distillation work? 3/3 Distillation columns are classified by the manner in which they are operated: 1. Batch, in which the feed to the column is introduced batch-wise. That is, the column is charged with a 'batch' and then the distillation process is carried out. When the desired task is achieved, a next batch of feed is introduced. 2. Continuous columns process a continuous feed stream. No interruptions occur unless there is a problem with the column or surrounding process units. They are capable of handling high throughputs and are the most common of the two types.

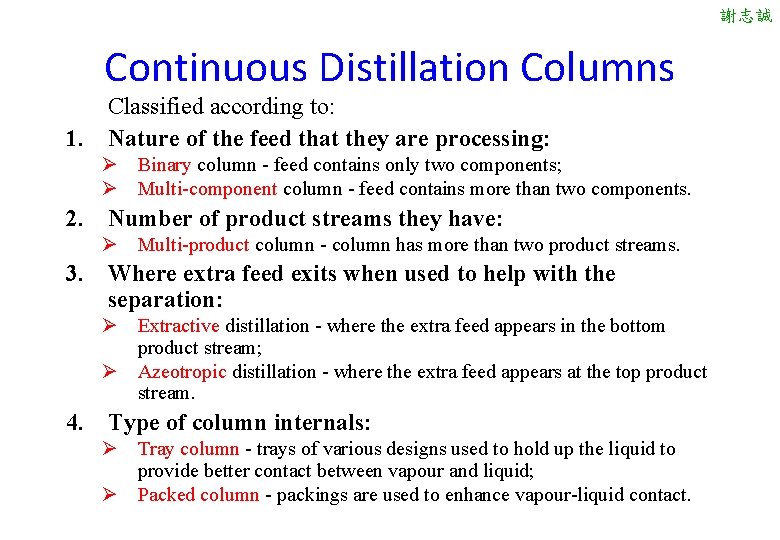
謝志誠 Continuous Distillation Columns Classified according to: 1. Nature of the feed that they are processing: Ø Binary column - feed contains only two components; Ø Multi-component column - feed contains more than two components. 2. Number of product streams they have: Ø Multi-product column - column has more than two product streams. 3. Where extra feed exits when used to help with the separation: Ø Extractive distillation - where the extra feed appears in the bottom product stream; Ø Azeotropic distillation - where the extra feed appears at the top product stream. 4. Type of column internals: Ø Tray column - trays of various designs used to hold up the liquid to provide better contact between vapour and liquid; Ø Packed column - packings are used to enhance vapour-liquid contact.

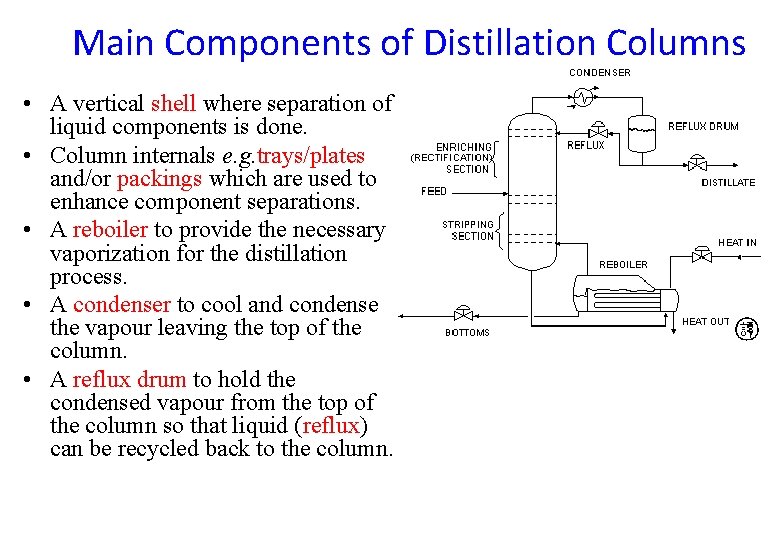
Main Components of Distillation Columns • A vertical shell where separation of liquid components is done. • Column internals e. g. trays/plates and/or packings which are used to enhance component separations. • A reboiler to provide the necessary vaporization for the distillation process. • A condenser to cool and condense the vapour leaving the top of the column. • A reflux drum to hold the condensed vapour from the top of the column so that liquid (reflux) can be recycled back to the column.

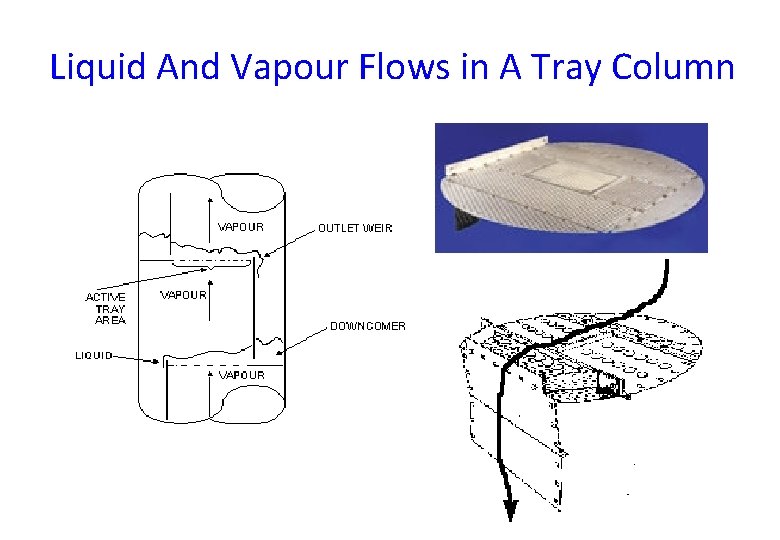
Liquid And Vapour Flows in A Tray Column

Trays and Plates 1/2 Bubble cap trays A riser or chimney is fitted over each hole, and a cap covers the riser. The cap is mounted with a space to allow vapour to rise through the chimney and be directed downward by the cap, finally discharging through slots in the cap, and bubbling through the liquid on the tray.

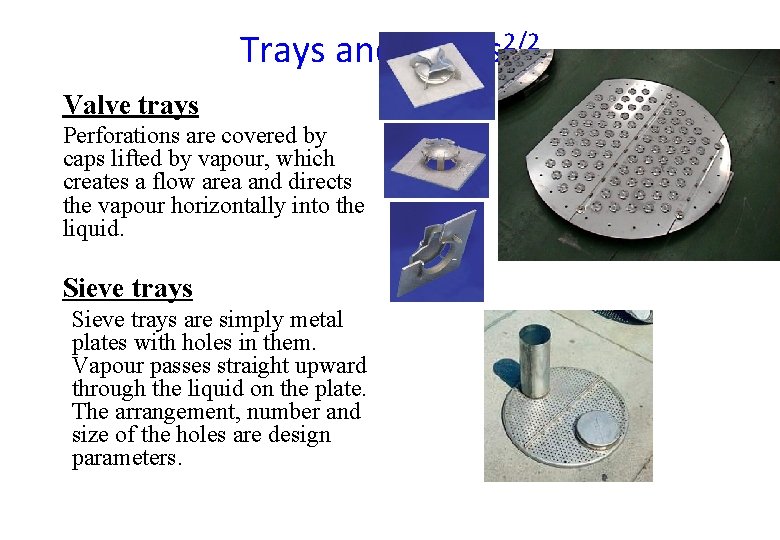
Trays and Plates 2/2 Valve trays Perforations are covered by caps lifted by vapour, which creates a flow area and directs the vapour horizontally into the liquid. Sieve trays are simply metal plates with holes in them. Vapour passes straight upward through the liquid on the plate. The arrangement, number and size of the holes are design parameters.

Packings • Packings are passive devices designed to increase the interfacial area for vapour-liquid contact. • They do not cause excessive pressure-drop across a packed section, which is important because a high pressure drop would mean that more energy is required to drive the vapour up the distillation column. • Packed columns are called continuous-contact columns while trayed columns are called staged-contact columns because of the manner in which vapour and liquid are contacted.

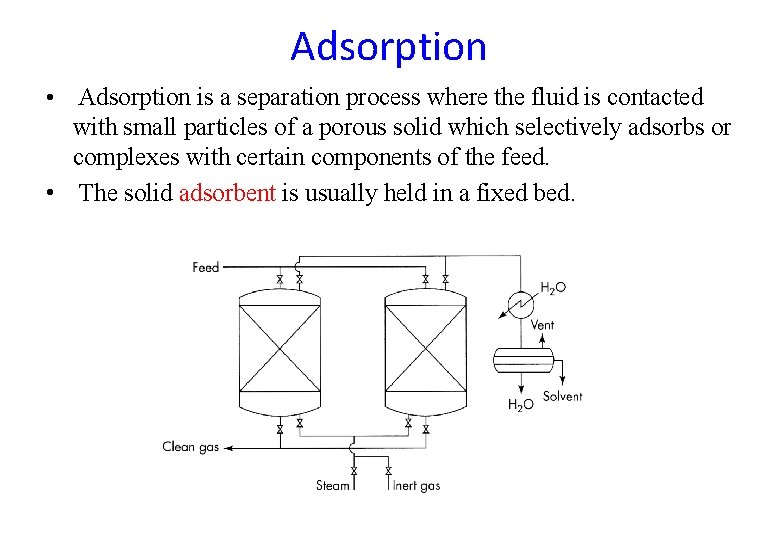
Adsorption • Adsorption is a separation process where the fluid is contacted with small particles of a porous solid which selectively adsorbs or complexes with certain components of the feed. • The solid adsorbent is usually held in a fixed bed.

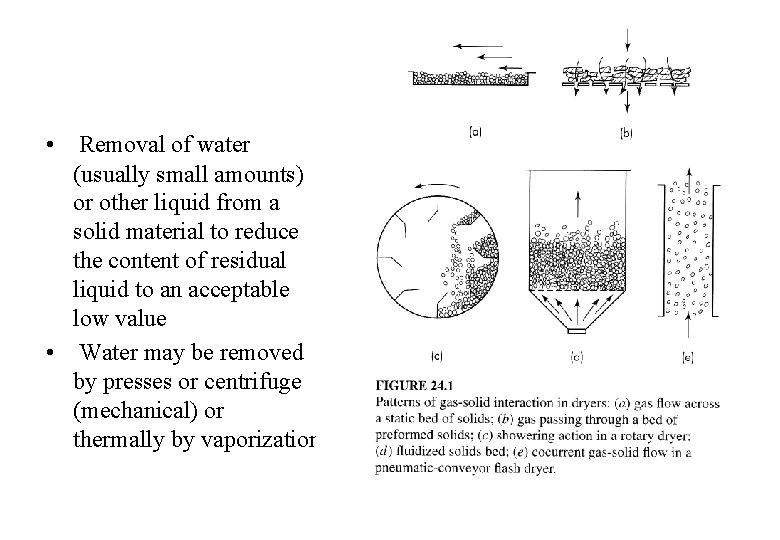
Drying • Removal of water (usually small amounts) or other liquid from a solid material to reduce the content of residual liquid to an acceptable low value • Water may be removed by presses or centrifuge (mechanical) or thermally by vaporization

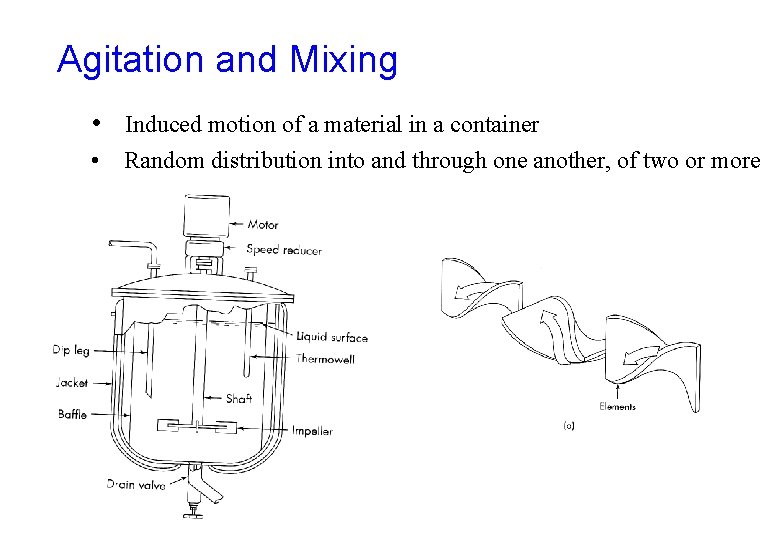
Agitation and Mixing • Induced motion of a material in a container • Random distribution into and through one another, of two or more initially separate phases.

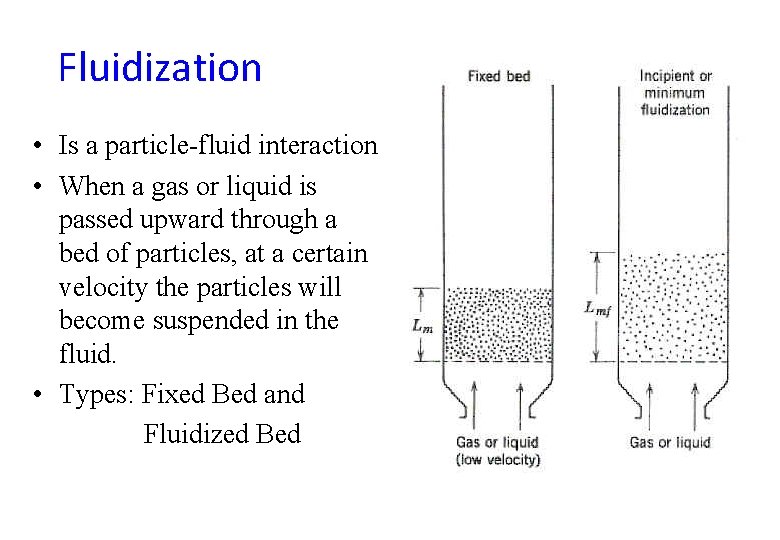
Fluidization • Is a particle-fluid interaction • When a gas or liquid is passed upward through a bed of particles, at a certain velocity the particles will become suspended in the fluid. • Types: Fixed Bed and Fluidized Bed

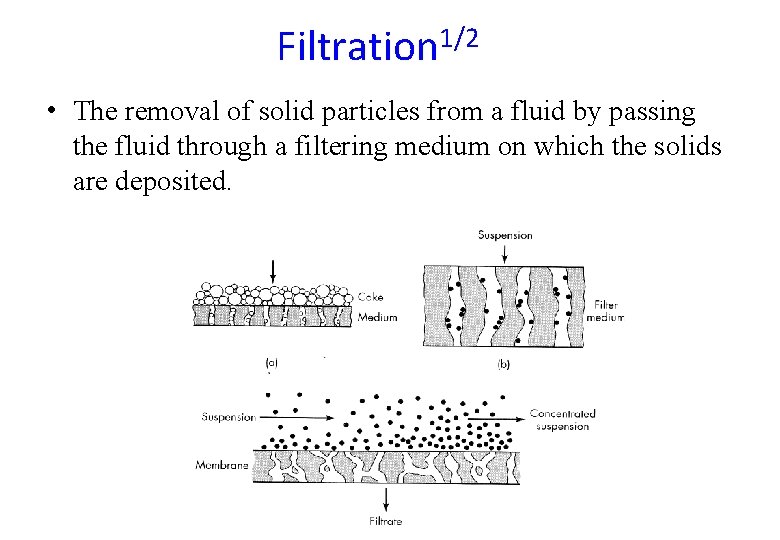
Filtration 1/2 • The removal of solid particles from a fluid by passing the fluid through a filtering medium on which the solids are deposited.

Filtration 2/2

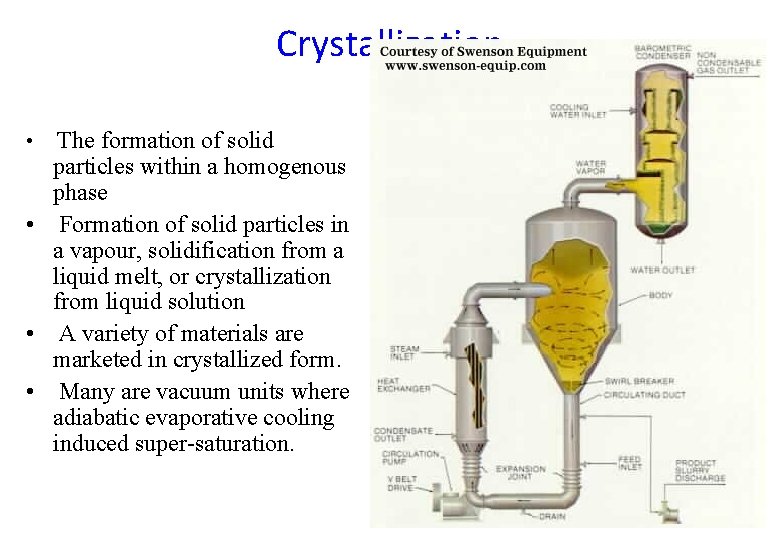
Crystallization The formation of solid particles within a homogenous phase • Formation of solid particles in a vapour, solidification from a liquid melt, or crystallization from liquid solution • A variety of materials are marketed in crystallized form. • Many are vacuum units where adiabatic evaporative cooling induced super-saturation. •

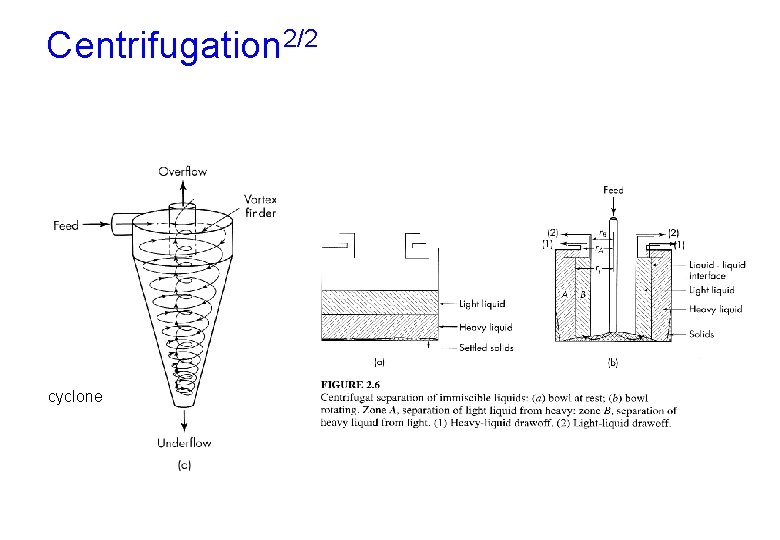
Centrifugation 1/2 • Many given particles settle under gravitational force at a fixed maximum rate • To increase the settling rate we replace the force of gravity by a much stronger centrifugal force. • More effective than gravity separators because they will separate fine drops and particles and are much smaller in size for a given capacity. • Cyclones: used for solids removal from gas:

Centrifugation 2/2 cyclone

Materials handling • Materials handling ØClassification: • Characterization of solids by size and shape • Done in a series of standard screens or woven wire test sieves arranged serially on a stack, with the smallest mesh on the bottom and the largest on top. ØComminution • Size reduction of solid particles , for example, chunks of coke must be reduced to workable size • Compression, impact, attrition (rubbing) or cutting, crushers and grinders are good examples.

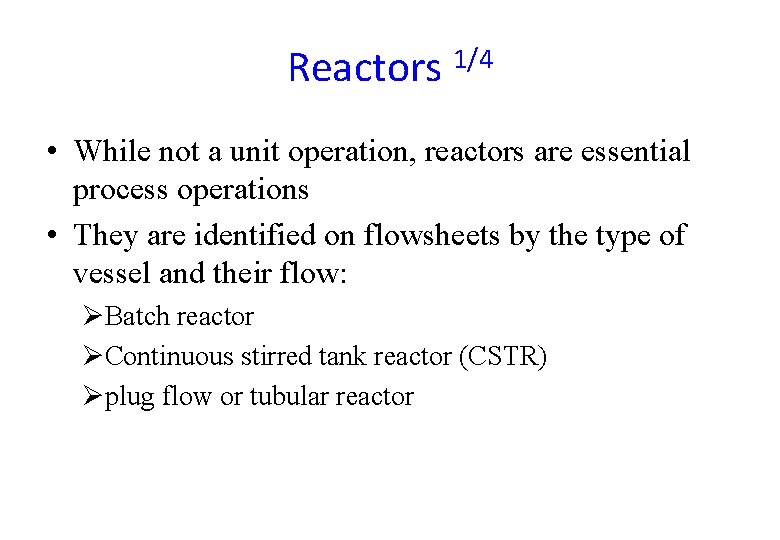
Reactors 1/4 • While not a unit operation, reactors are essential process operations • They are identified on flowsheets by the type of vessel and their flow: ØBatch reactor ØContinuous stirred tank reactor (CSTR) Øplug flow or tubular reactor

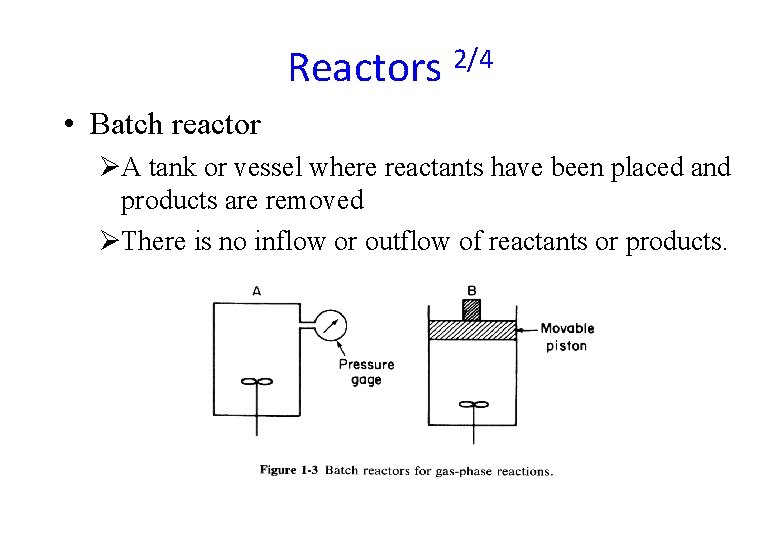
Reactors 2/4 • Batch reactor ØA tank or vessel where reactants have been placed and products are removed ØThere is no inflow or outflow of reactants or products.

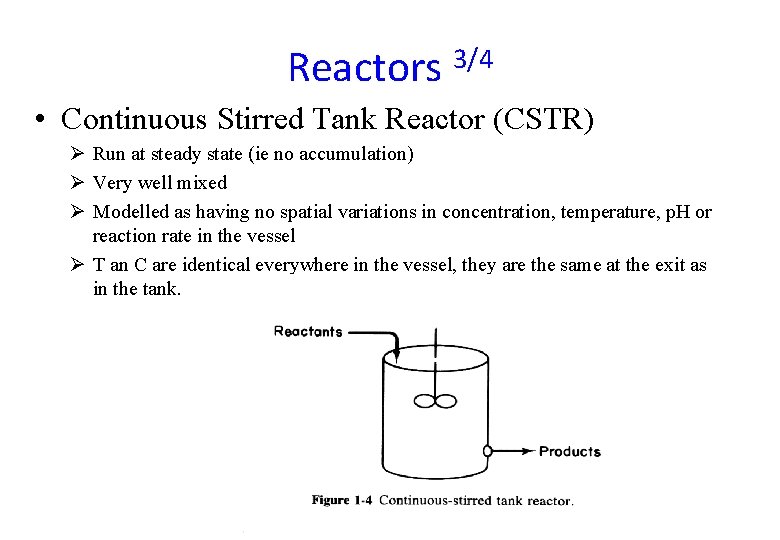
Reactors 3/4 • Continuous Stirred Tank Reactor (CSTR) Ø Run at steady state (ie no accumulation) Ø Very well mixed Ø Modelled as having no spatial variations in concentration, temperature, p. H or reaction rate in the vessel Ø T an C are identical everywhere in the vessel, they are the same at the exit as in the tank.

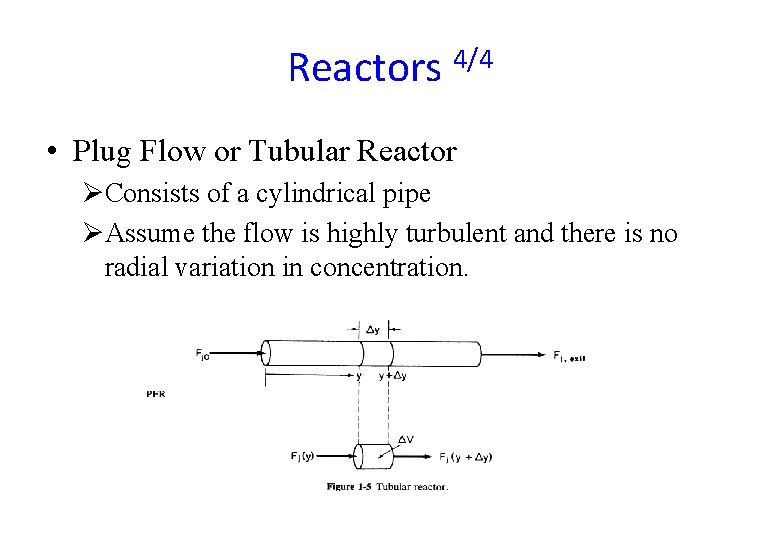
Reactors 4/4 • Plug Flow or Tubular Reactor ØConsists of a cylindrical pipe ØAssume the flow is highly turbulent and there is no radial variation in concentration.

謝志誠 End of Chapter 1