Chemical Potential Enthalpy H entropy S and Gibbs
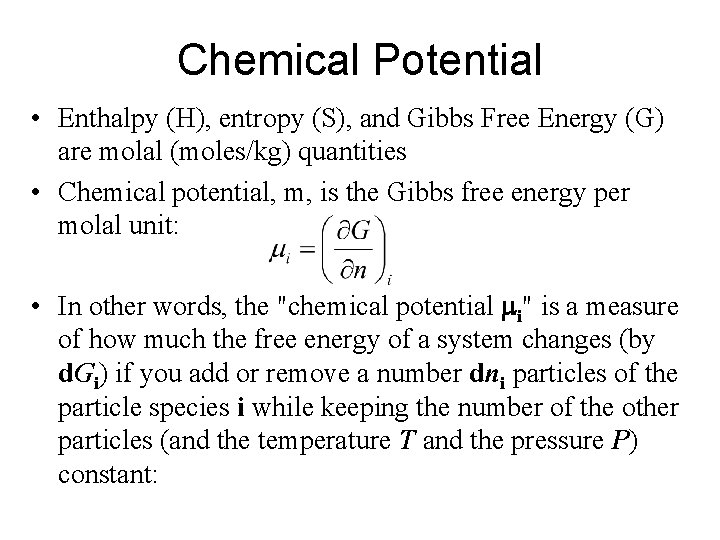
Chemical Potential • Enthalpy (H), entropy (S), and Gibbs Free Energy (G) are molal (moles/kg) quantities • Chemical potential, m, is the Gibbs free energy per molal unit: • In other words, the "chemical potential mi" is a measure of how much the free energy of a system changes (by d. Gi) if you add or remove a number dni particles of the particle species i while keeping the number of the other particles (and the temperature T and the pressure P) constant:

Mixing • Putting two components into the same system – they mix and potentially interact: • Mechanical mixture – no chemical interaction: where X is mole fraction of A, B ms = X A m. A + X B m. B • Random mixture – particles spontaneously (so m must go down) orient randomly: Dmmix=ms – mmechanical mixing Mixing ideal IF interaction of A-A = A-B = B-B if that is true then DHmix=0, so DSmix must be >0 (because mmix<0 (spontaneous mixing): R=molar gas constant DSid mix = -RSXiln. Xi X=mole fraction component i
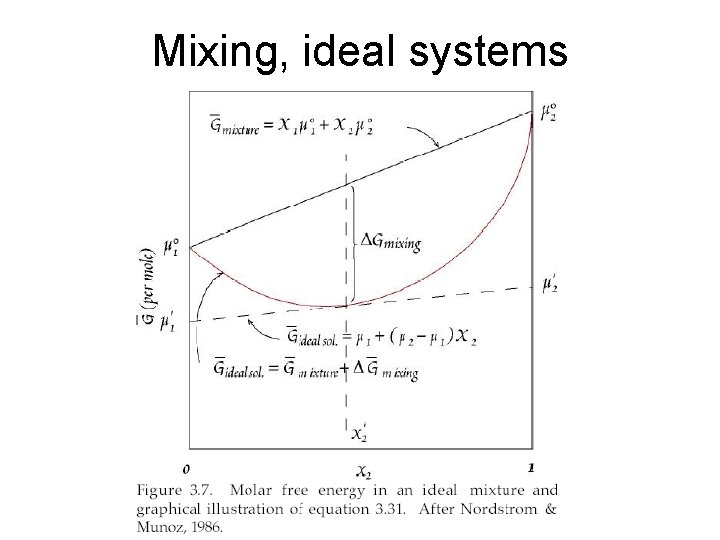
Mixing, ideal systems
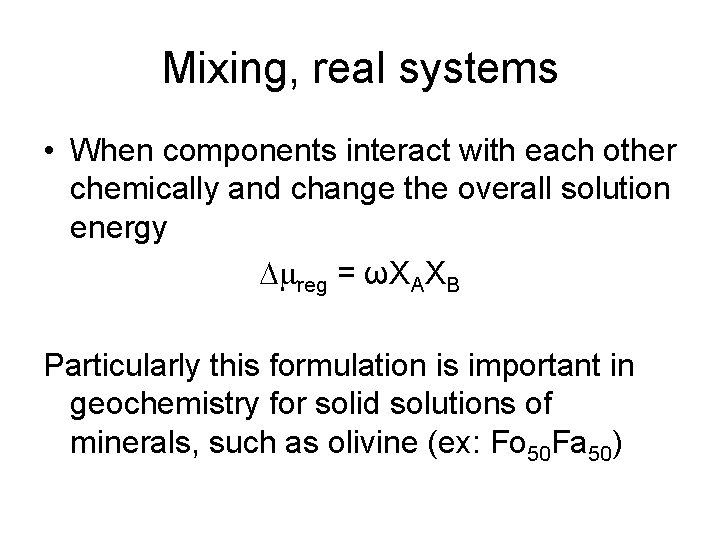
Mixing, real systems • When components interact with each other chemically and change the overall solution energy Dmreg = ωXAXB Particularly this formulation is important in geochemistry for solid solutions of minerals, such as olivine (ex: Fo 50 Fa 50)

Mixing, a more complete picture Energy = mechanical mixture + ideal mixing + regular solution Put 2 things together, disperse them, then they interact… mtot= XAm 0 A+(1 -XA)m 0 B + XARTln. XA+ (1 XA)RTln(1 -XA) + ωXA(1 -XA)

Mixing and miscibility • What about systems where phases do not mix (oil and water)? ?

P-X stability and mixing
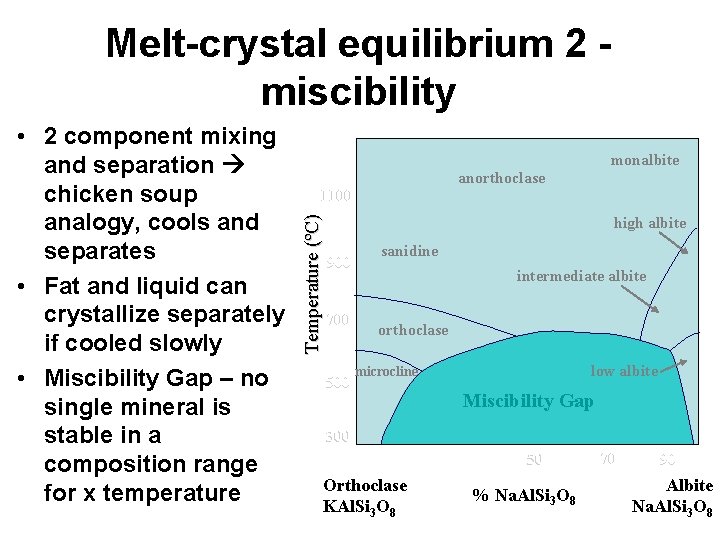
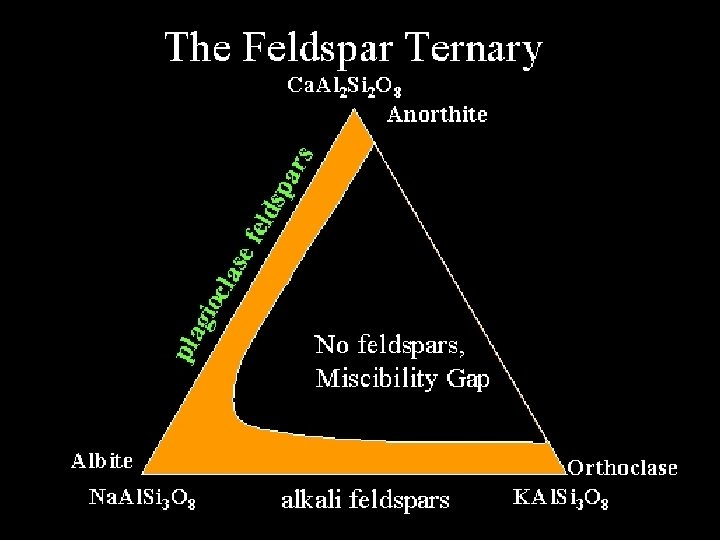
Melt-crystal equilibrium 2 miscibility monalbite anorthoclase 1100 Temperature (ºC) • 2 component mixing and separation chicken soup analogy, cools and separates • Fat and liquid can crystallize separately if cooled slowly • Miscibility Gap – no single mineral is stable in a composition range for x temperature high albite 900 700 500 sanidine intermediate albite orthoclase low albite microcline Miscibility Gap 300 10 Orthoclase KAl. Si 3 O 8 30 50 % Na. Al. Si 3 O 8 70 90 Albite Na. Al. Si 3 O 8
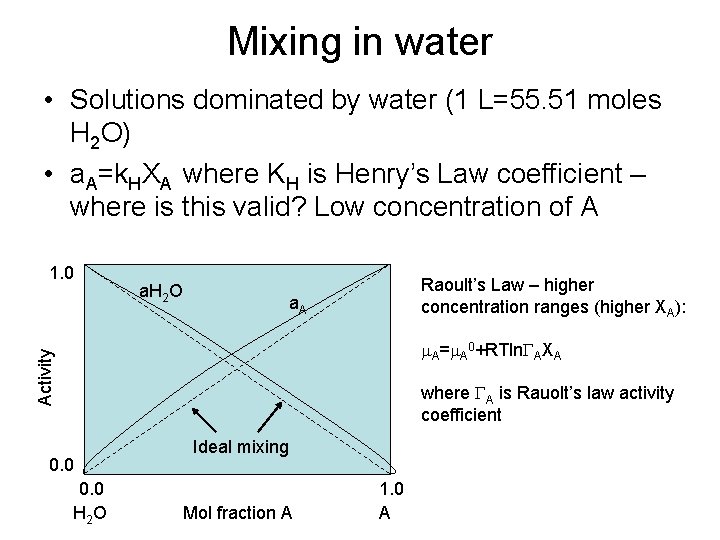
Mixing in water • Solutions dominated by water (1 L=55. 51 moles H 2 O) • a. A=k. HXA where KH is Henry’s Law coefficient – where is this valid? Low concentration of A 1. 0 a. H 2 O Raoult’s Law – higher concentration ranges (higher XA): a. A Activity m. A=m. A 0+RTln. GAXA where GA is Rauolt’s law activity coefficient 0. 0 H 2 O Ideal mixing Mol fraction A 1. 0 A
- Slides: 10