Chemical Physical Changes Properties of Elements Every element




- Slides: 9

Chemical & Physical Changes

Properties of Elements • Every element has properties that are unique to that element. • Though some elements have similar properties, no two elements have the exact same set of characteristics. • Properties can be classified as either Physical or Chemical

Physical Properties- a property that can be measured without altering the identity of the substance. These properties can be observed when the substance is by itself (not reacting with other substances). Examples: • Appearance (Shape, size, luster etc) • Conductivity (Heat or electricity) • Density • Malleability

Chemical Properties- a property that is only apparent when the substance undergoes a chemical reaction. These properties can only be observed when the substance is undergoing a chemical change. Examples: • Ability to Rust • Combustibility • Flammability • Reacts w/ Acid

Physical Changes • A change that involves changes of state or form of matter, but does not change chemical composition. • No new substances formed! • Ex – melting, boiling, freezing, tearing, ripping, cutting, heating etc: • Creating Solutions = Physical

Physical Changes - Solutions • A specific type of physical mixture. Homogenous mixture of two or more substances. • Homogenous = can’t tell substances apart. • Still Physical Change! • Ex – Salt Water, Kool-Aid

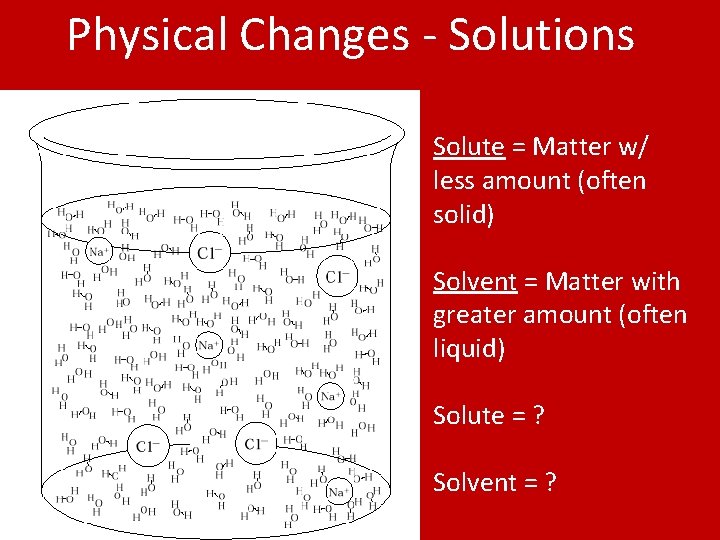
Physical Changes - Solutions Solute = Matter w/ less amount (often solid) Solvent = Matter with greater amount (often liquid) Solute = ? Solvent = ?

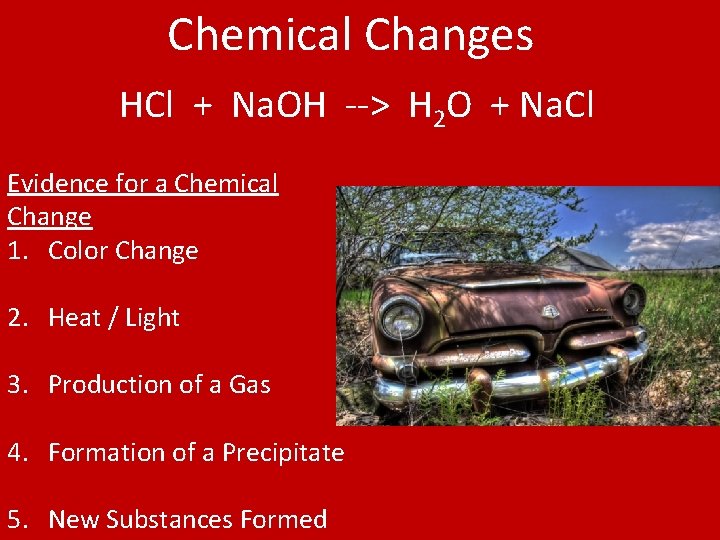
Chemical Changes Chemical Change • A change that creates new substances. New substances have different chemical composition than starting material. • Reactants = starting substances • Products = ending substances • Ex – fire, burning matches, rusting, acids/base reactions, digestion

Chemical Changes HCl + Na. OH --> H 2 O + Na. Cl Evidence for a Chemical Change 1. Color Change 2. Heat / Light 3. Production of a Gas 4. Formation of a Precipitate 5. New Substances Formed