Chemical Oxidation of Poly and Perfluoroalkyl Substances in

Chemical Oxidation of Poly- and Perfluoroalkyl Substances in AFFFImpacted Groundwater Thomas Bruton, David Sedlak Department of Civil and Environmental Engineering University of California at Berkeley
Poly- and Perfluoroalkyl Substances (PFAS) • Contain perfluoroalkyl functional group: Cn. F 2 n+1 – R • Chemical and thermal stability • Hydrophobic and lipophobic • Applications: • • • textile stain and soil repellents grease-proof food packaging non-stick cookware chemicals manufacturing aqueous film–forming foam (AFFF) used to extinguish fires involving flammable liquids 2

Poly- and Perfluoroalkyl Substances (PFAS) Perfluoroalkyl substances Perfluoroalkyl acids (PFAAs) Perfluorinated carboxylic acids (PFCAs) Polyfluoroalkyl substances Polymers Fluorotelomer-based compounds Perfluoroalkane sulfonamido derivatives Perfluorinated sulfonic acids (PFSAs) After Buck, et al. , Int. Env. Ass. Mgmnt, 2011 3
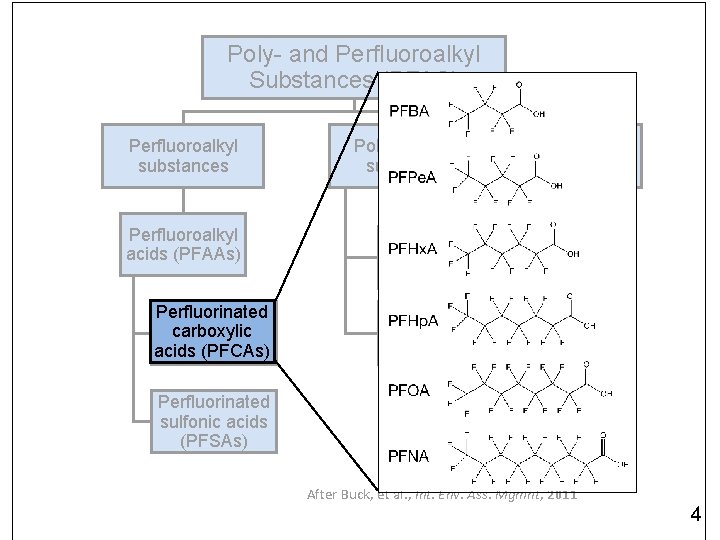
Poly- and Perfluoroalkyl Substances (PFAS) Perfluoroalkyl substances Perfluoroalkyl acids (PFAAs) Perfluorinated carboxylic acids (PFCAs) Polyfluoroalkyl substances Polymers Fluorotelomer-based compounds Perfluoroalkane sulfonamido derivatives Perfluorinated sulfonic acids (PFSAs) After Buck, et al. , Int. Env. Ass. Mgmnt, 2011 4
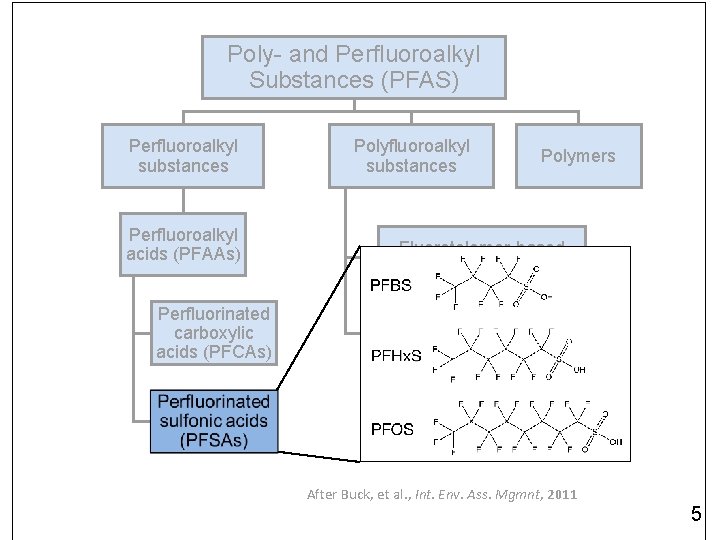
Poly- and Perfluoroalkyl Substances (PFAS) Perfluoroalkyl substances Perfluoroalkyl acids (PFAAs) Perfluorinated carboxylic acids (PFCAs) Polyfluoroalkyl substances Polymers Fluorotelomer-based compounds Perfluoroalkane sulfonamido derivatives Perfluorinated sulfonic acids (PFSAs) After Buck, et al. , Int. Env. Ass. Mgmnt, 2011 5
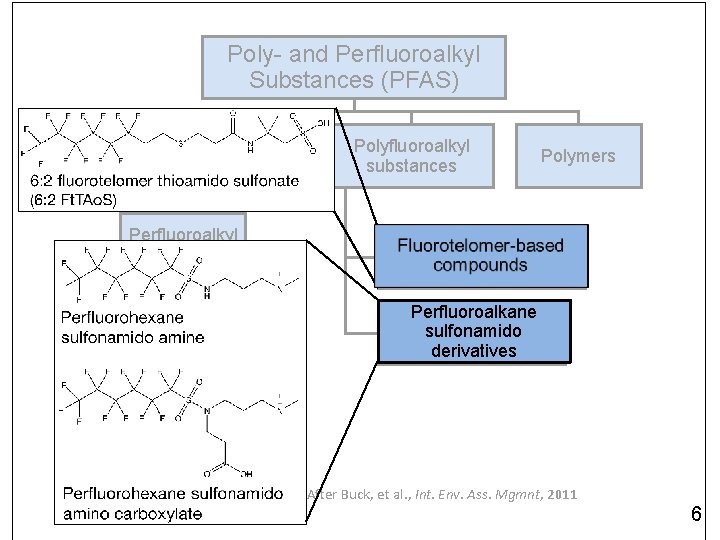
Poly- and Perfluoroalkyl Substances (PFAS) Perfluoroalkyl substances Perfluoroalkyl acids (PFAAs) Perfluorinated carboxylic acids (PFCAs) Polyfluoroalkyl substances Polymers Fluorotelomer-based compounds Perfluoroalkane sulfonamido derivatives Perfluorinated sulfonic acids (PFSAs) After Buck, et al. , Int. Env. Ass. Mgmnt, 2011 6
PFOA and PFOS • Persistent • Bioaccumulative • Toxic • • • developmental effects cancer liver damage immune system effects thyroid effects • U. S. EPA Lifetime Health Advisory • 70 ppt combined (May 2016) 7
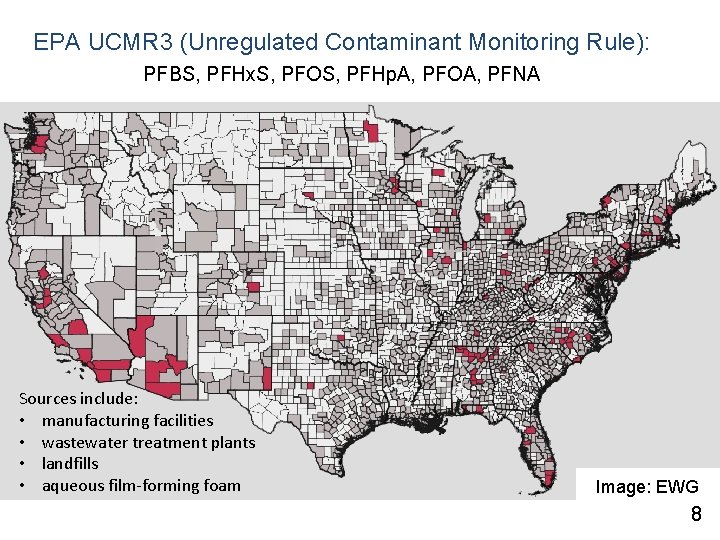
EPA UCMR 3 (Unregulated Contaminant Monitoring Rule): PFBS, PFHx. S, PFOS, PFHp. A, PFOA, PFNA Sources include: • manufacturing facilities • wastewater treatment plants • landfills • aqueous film-forming foam Image: EWG 8
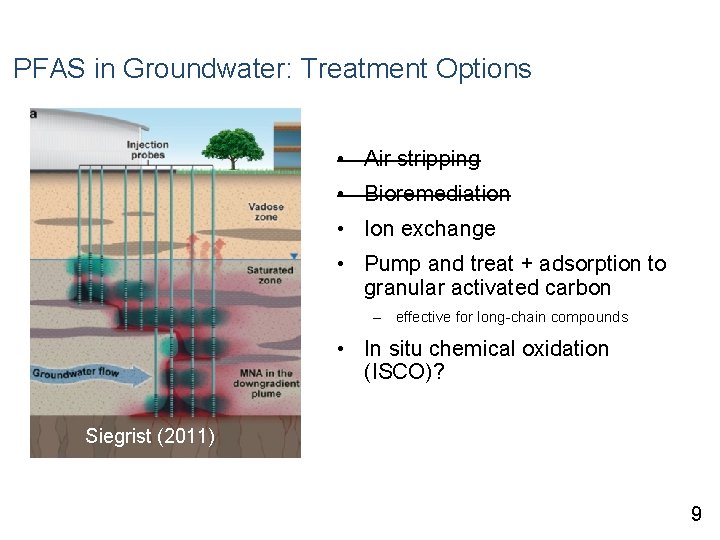
PFAS in Groundwater: Treatment Options • Air stripping • Bioremediation • Ion exchange • Pump and treat + adsorption to granular activated carbon – effective for long-chain compounds • In situ chemical oxidation (ISCO)? Siegrist (2011) 9
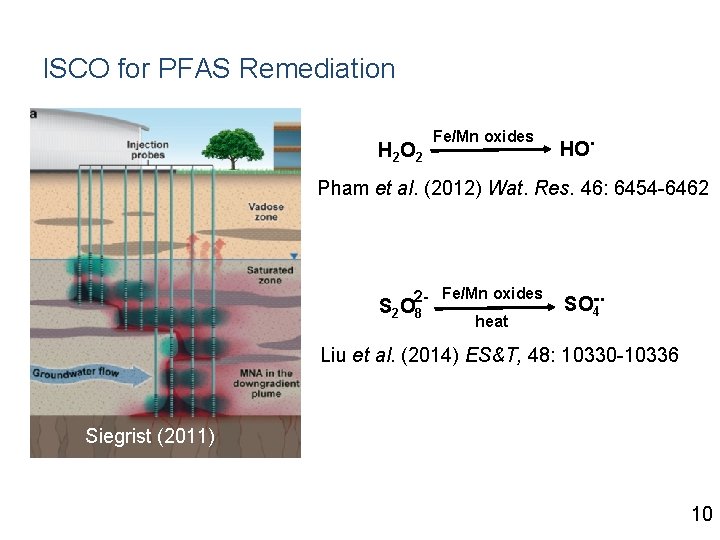
ISCO for PFAS Remediation H 2 O 2 Fe/Mn oxides HO . Pham et al. (2012) Wat. Res. 46: 6454 -6462 2 - Fe/Mn oxides S 2 O 8 heat . SO-4 Liu et al. (2014) ES&T, 48: 10330 -10336 Siegrist (2011) 10
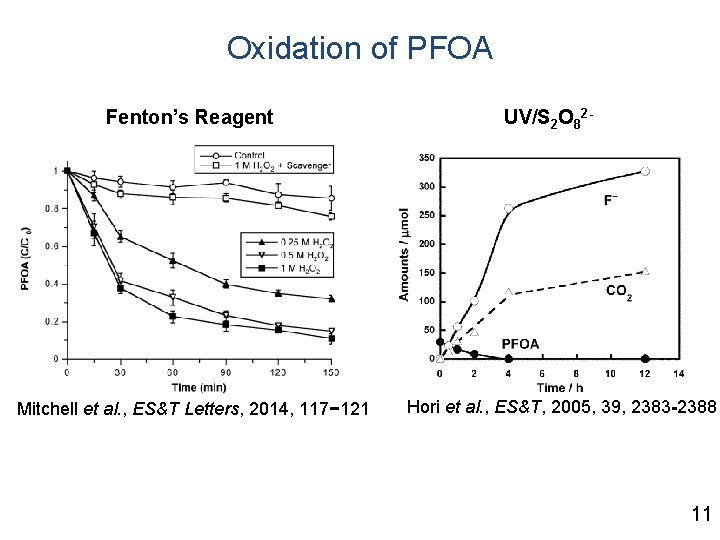
Oxidation of PFOA Fenton’s Reagent Mitchell et al. , ES&T Letters, 2014, 117− 121 UV/S 2 O 82 - Hori et al. , ES&T, 2005, 39, 2383 -2388 11
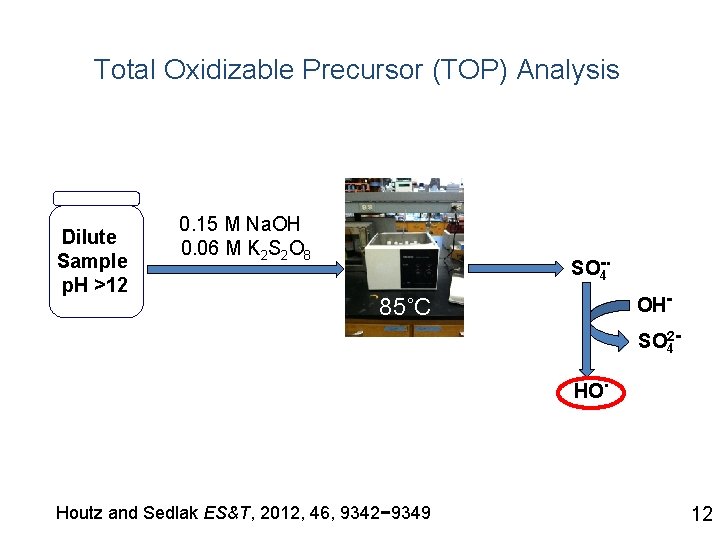
Total Oxidizable Precursor (TOP) Analysis Dilute Sample p. H >12 0. 15 M Na. OH 0. 06 M K 2 S 2 O 8 . SO-4 OH- 85˚C SO 24 HO Houtz and Sedlak ES&T, 2012, 46, 9342− 9349 . 12
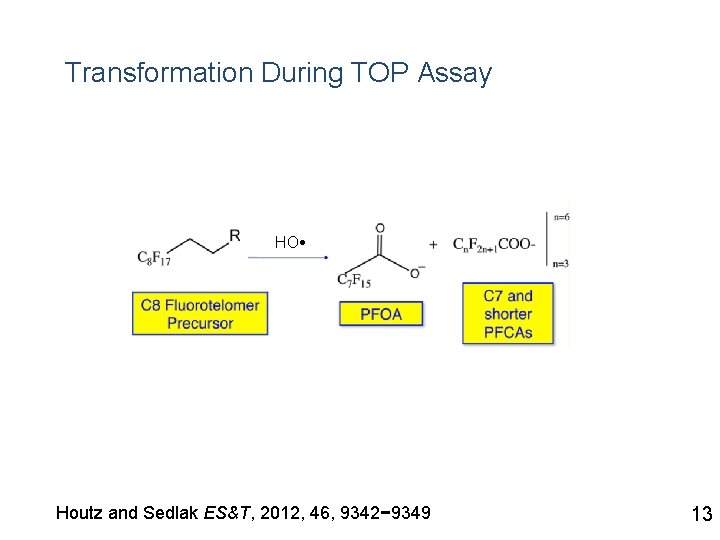
Transformation During TOP Assay HO Houtz and Sedlak ES&T, 2012, 46, 9342− 9349 13

Example: Conversion of AFFF Before Oxidation After Oxidation No carboxylates PFNA (C 9) detected PFOA (C 8) Ansul 19841987 Ansul 20082010 PFHp. A (C 7) Buckeye 2009 PFPe. A (C 5) PFHx. A (C 6) PFBA (C 4) National Foam 2002 -2008 0 1 2 3 4 Concentration, g/L Ansul 19841987 PFNA (C 9) Ansul 20082010 PFHp. A (C 7) Buckeye 2009 PFPe. A (C 5) PFOA (C 8) PFHx. A (C 6) PFBA (C 4) National Foam 2002 -2008 5 Houtz et al. ES&T, 2013, 46: 9342 -9349. 0 1 2 3 4 Concentration, g/L 5 14
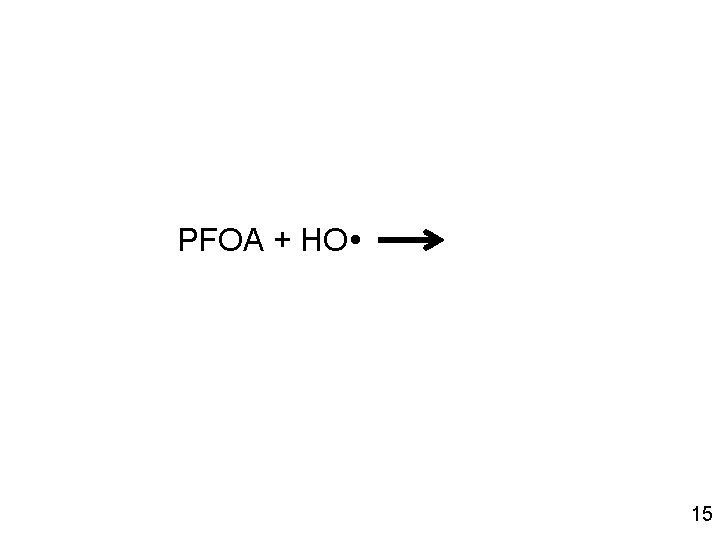
PFOA + HO 15

PFOA + HO 15
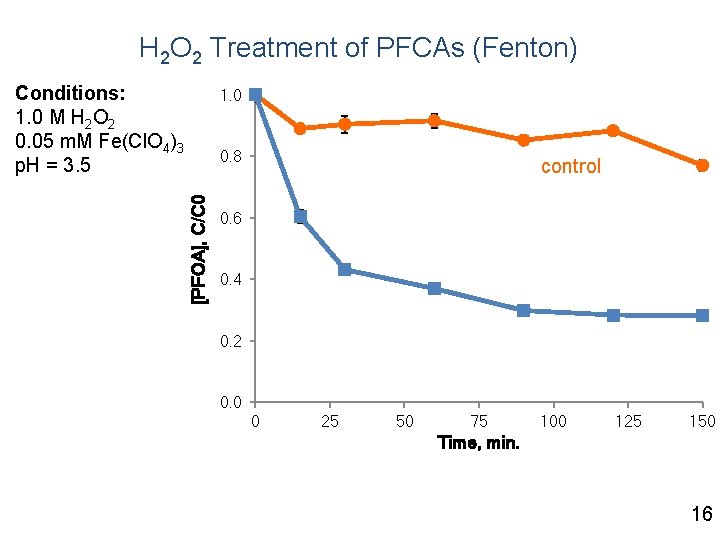
H 2 O 2 Treatment of PFCAs (Fenton) Conditions: 1. 0 M H 2 O 2 0. 05 m. M Fe(Cl. O 4)3 p. H = 3. 5 1. 0 [PFOA], C/C 0 0. 8 control 0. 6 0. 4 0. 2 0. 0 0 25 50 75 100 125 150 Time, min. 16
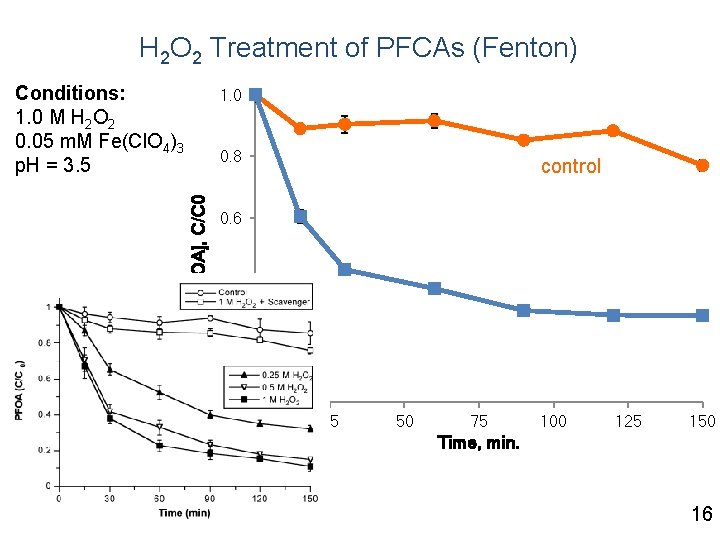
H 2 O 2 Treatment of PFCAs (Fenton) Conditions: 1. 0 M H 2 O 2 0. 05 m. M Fe(Cl. O 4)3 p. H = 3. 5 1. 0 [PFOA], C/C 0 0. 8 control 0. 6 0. 4 0. 2 0. 0 0 25 50 75 100 125 150 Time, min. 16
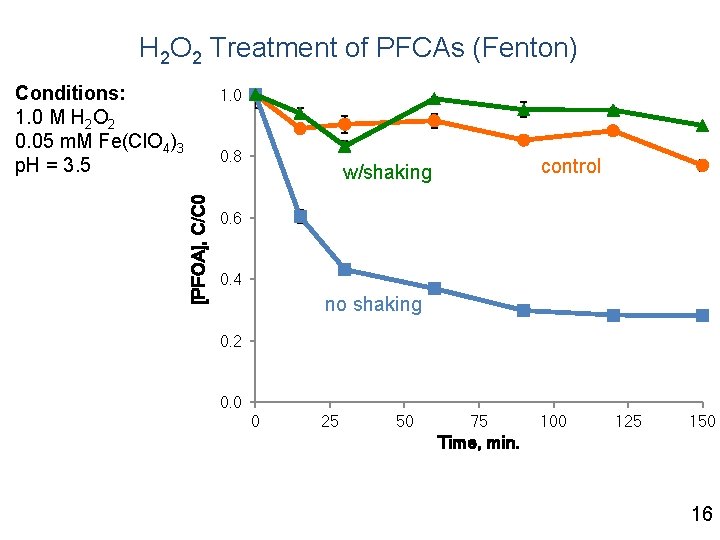
H 2 O 2 Treatment of PFCAs (Fenton) Conditions: 1. 0 M H 2 O 2 0. 05 m. M Fe(Cl. O 4)3 p. H = 3. 5 1. 0 [PFOA], C/C 0 0. 8 control w/shaking 0. 6 0. 4 no shaking 0. 2 0. 0 0 25 50 75 100 125 150 Time, min. 16
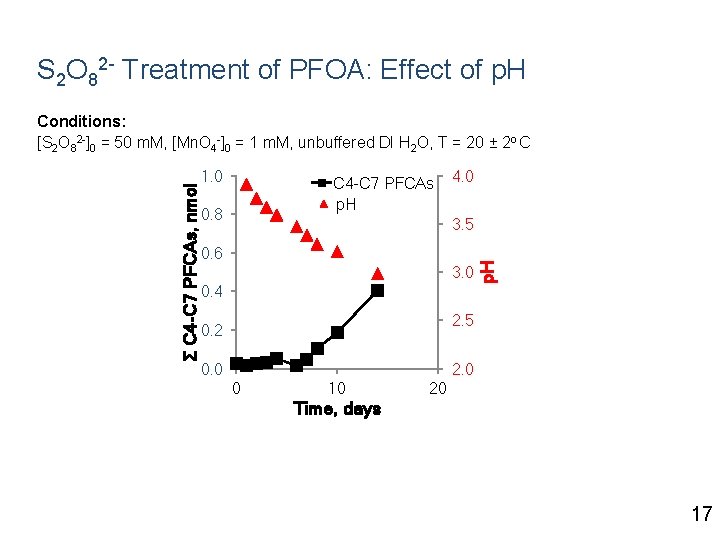
S 2 O 82 - Treatment of PFOA: Effect of p. H 1. 0 C 4 -C 7 PFCAs p. H 0. 8 4. 0 3. 5 0. 6 3. 0 0. 4 p. H Σ C 4 -C 7 PFCAs, nmol Conditions: [S 2 O 82 -]0 = 50 m. M, [Mn. O 4 -]0 = 1 m. M, unbuffered DI H 2 O, T = 20 ± 2 o C 2. 5 0. 2 0. 0 2. 0 0 10 20 Time, days 17
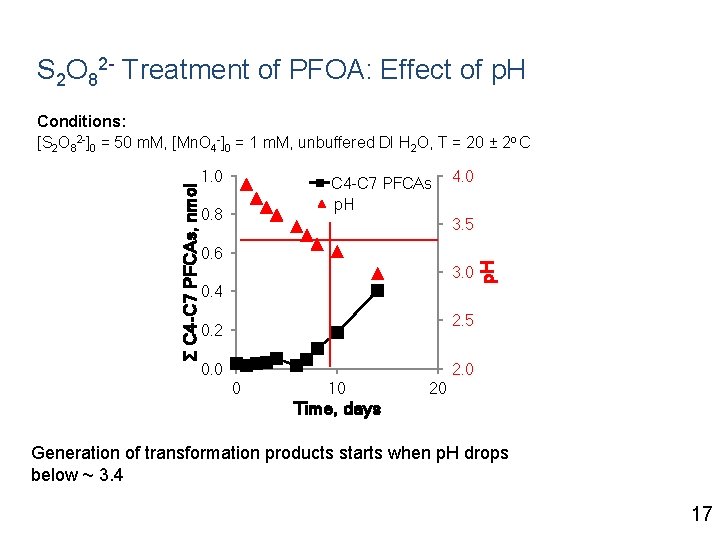
S 2 O 82 - Treatment of PFOA: Effect of p. H 1. 0 C 4 -C 7 PFCAs p. H 0. 8 4. 0 3. 5 0. 6 3. 0 0. 4 p. H Σ C 4 -C 7 PFCAs, nmol Conditions: [S 2 O 82 -]0 = 50 m. M, [Mn. O 4 -]0 = 1 m. M, unbuffered DI H 2 O, T = 20 ± 2 o C 2. 5 0. 2 0. 0 2. 0 0 10 20 Time, days Generation of transformation products starts when p. H drops below ~ 3. 4 17
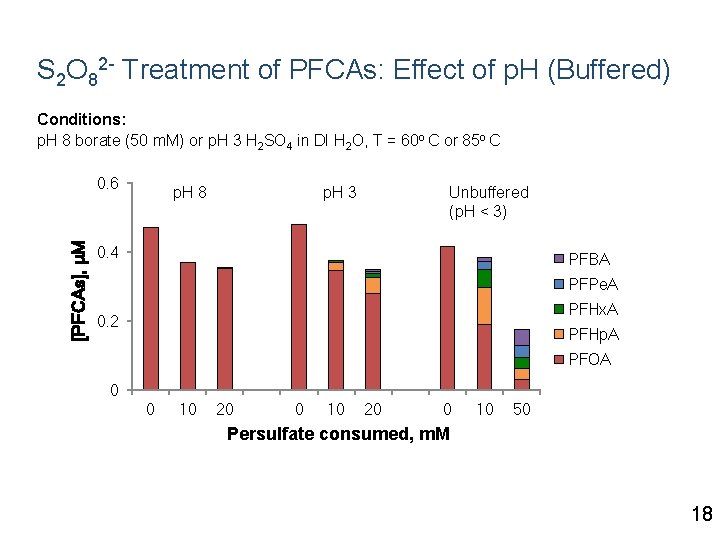
S 2 O 82 - Treatment of PFCAs: Effect of p. H (Buffered) Conditions: p. H 8 borate (50 m. M) or p. H 3 H 2 SO 4 in DI H 2 O, T = 60 o C or 85 o C [PFCAs], μM 0. 6 p. H 8 p. H 3 Unbuffered (p. H < 3) 0. 4 PFBA PFPe. A PFHx. A 0. 2 PFHp. A PFOA 0 0 10 20 0 10 50 Persulfate consumed, m. M 18
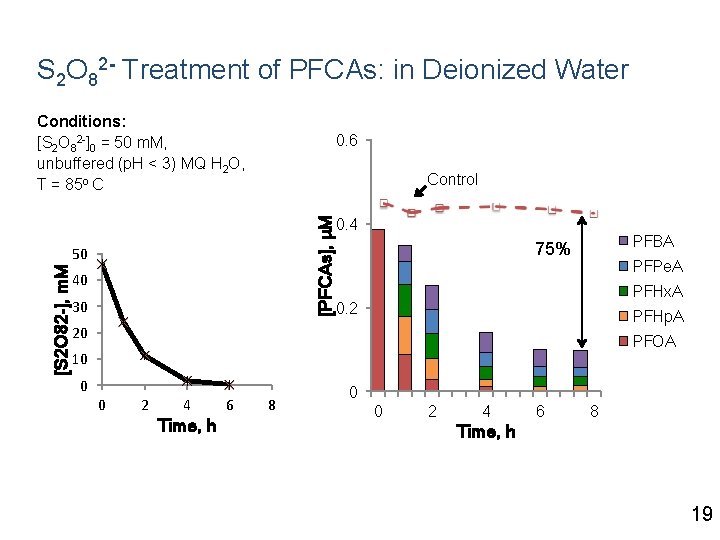
S 2 O 82 - Treatment of PFCAs: in Deionized Water Conditions: [S 2 O 82 -]0 = 50 m. M, unbuffered (p. H < 3) MQ H 2 O, T = 85 o C 0. 6 [PFCAs], μM Control [S 2 O 82 -], m. M 50 40 30 0. 4 PFBA 75% PFPe. A PFHx. A 0. 2 PFHp. A 20 PFOA 10 0 0 2 4 Time, h 6 8 0 0 2 4 6 8 Time, h 19
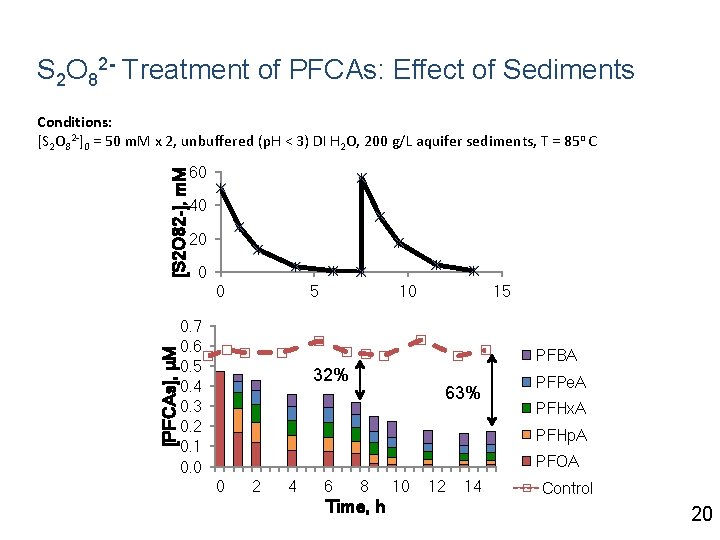
S 2 O 82 - Treatment of PFCAs: Effect of Sediments [S 2 O 82 -], m. M Conditions: [S 2 O 82 -]0 = 50 m. M x 2, unbuffered (p. H < 3) DI H 2 O, 200 g/L aquifer sediments, T = 85 o C 60 40 20 0 [PFCAs], μM 0 5 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 10 15 PFBA 32% 63% PFPe. A PFHx. A PFHp. A PFOA 0 2 4 6 8 Time, h 10 12 14 Control 20
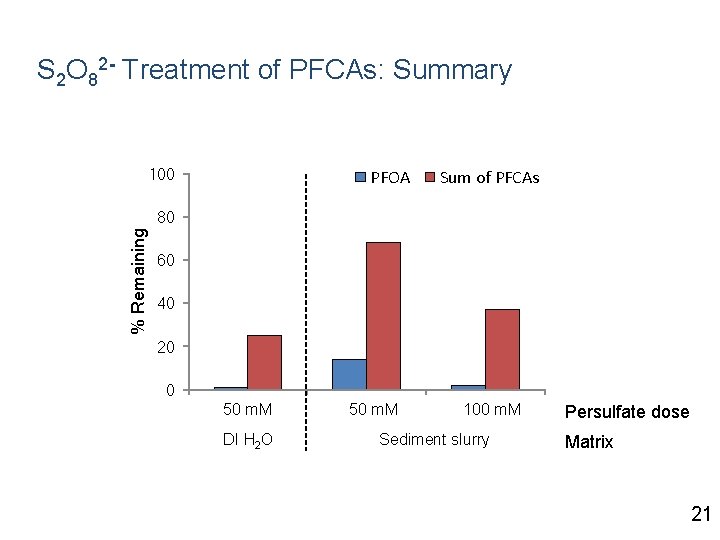
S 2 O 82 - Treatment of PFCAs: Summary 100 PFOA Sum of PFCAs % Remaining 80 60 40 20 0 50 m. M DI H 2 O 50 m. M 100 m. M Sediment slurry Persulfate dose Matrix 21

8
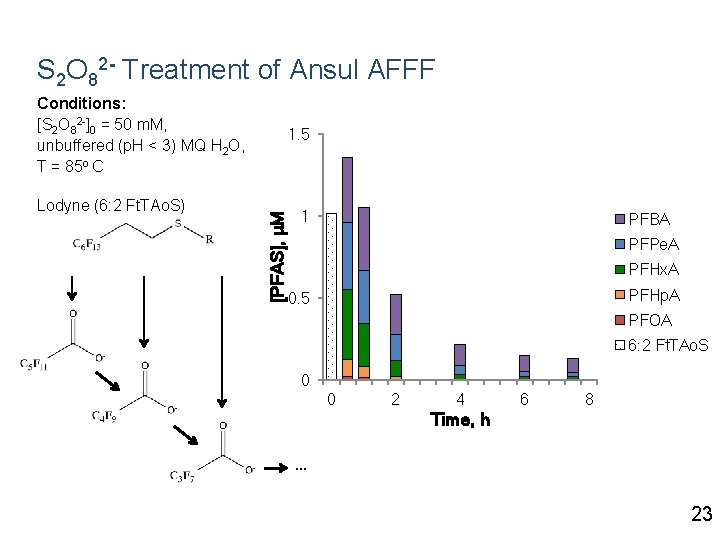
S 2 O 82 - Treatment of Ansul AFFF Conditions: [S 2 O 82 -]0 = 50 m. M, unbuffered (p. H < 3) MQ H 2 O, T = 85 o C [PFAS], μM Lodyne (6: 2 Ft. TAo. S) 1. 5 1 PFBA PFPe. A PFHx. A PFHp. A 0. 5 PFOA 6: 2 Ft. TAo. S 0 0 2 4 6 8 Time, h … 23
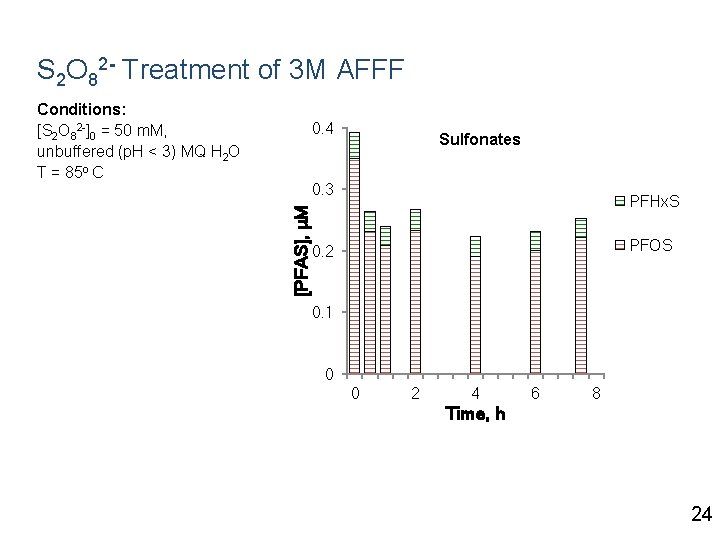
S 2 O 82 - Treatment of 3 M AFFF Conditions: [S 2 O 82 -]0 = 50 m. M, unbuffered (p. H < 3) MQ H 2 O T = 85 o C 0. 4 Sulfonates [PFAS], μM 0. 3 PFHx. S PFOS 0. 2 0. 1 0 0 2 4 6 8 Time, h 24
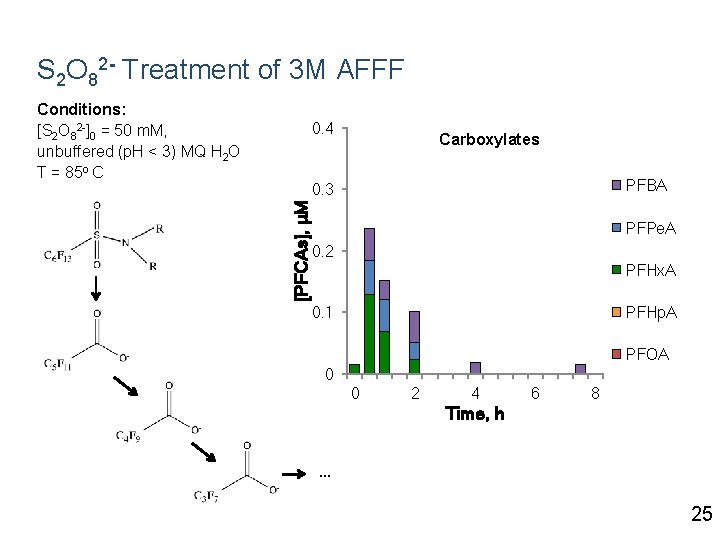
S 2 O 82 - Treatment of 3 M AFFF Conditions: [S 2 O 82 -]0 = 50 m. M, unbuffered (p. H < 3) MQ H 2 O T = 85 o C 0. 4 Carboxylates PFBA [PFCAs], μM 0. 3 PFPe. A 0. 2 PFHx. A PFHp. A 0. 1 PFOA 0 0 2 4 6 8 Time, h … 25
Summary Fenton - PFOA loss due to sorption, not oxidation Persulfate - conversion of precursors to PFCAs - degradation of PFCAs - p. H, sediment - not effective for PFSAs Research Needs - mechanistic explanation - pilot/demonstration-scale 26

Thanks to Jennifer Field Chris Higgins Lisa Alvarez-Cohen Erika Houtz Katie Harding-Marjanovic Shan Yi 27
- Slides: 31