Chemical Nomenclature Section 2 2 Nomenclature system used







- Slides: 30

Chemical Nomenclature Section 2. 2

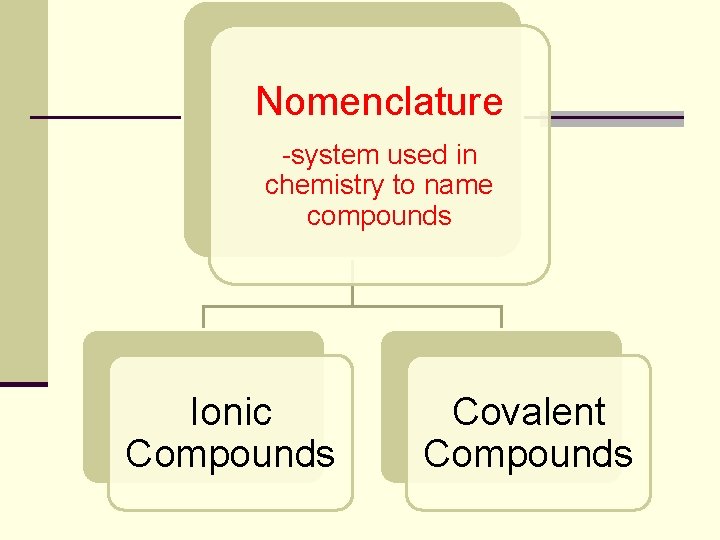
Nomenclature -system used in chemistry to name compounds Ionic Compounds Covalent Compounds

Naming Ionic Compounds n. Binary compounds and n. Polyatomic compounds

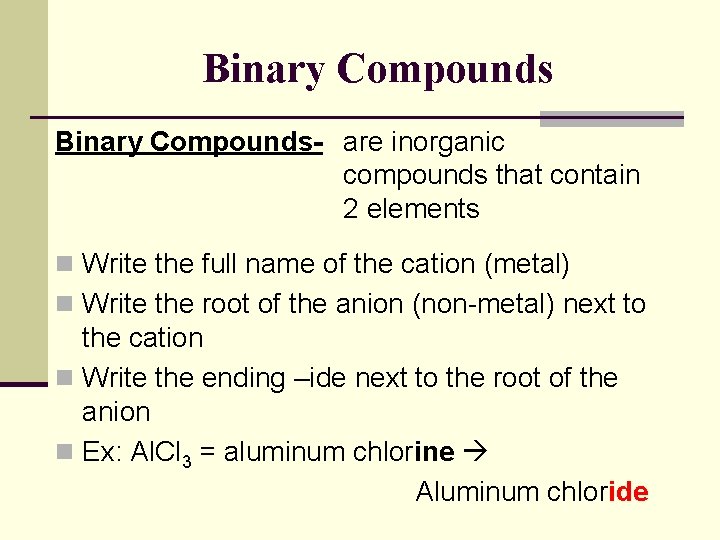
Binary Compounds- are inorganic compounds that contain 2 elements n Write the full name of the cation (metal) n Write the root of the anion (non-metal) next to the cation n Write the ending –ide next to the root of the anion n Ex: Al. Cl 3 = aluminum chlorine Aluminum chloride

Transition Metal Ions n Some elements may form more than one cation (ions with different charges), these are called multivalent ions. n You will indicate the charge of the ion by writing the charge in roman numerals after the cation name n Use the cross up rule to find charges! Ex: Fe 2 O 2 and Fe 2 O 3 _______ and _______

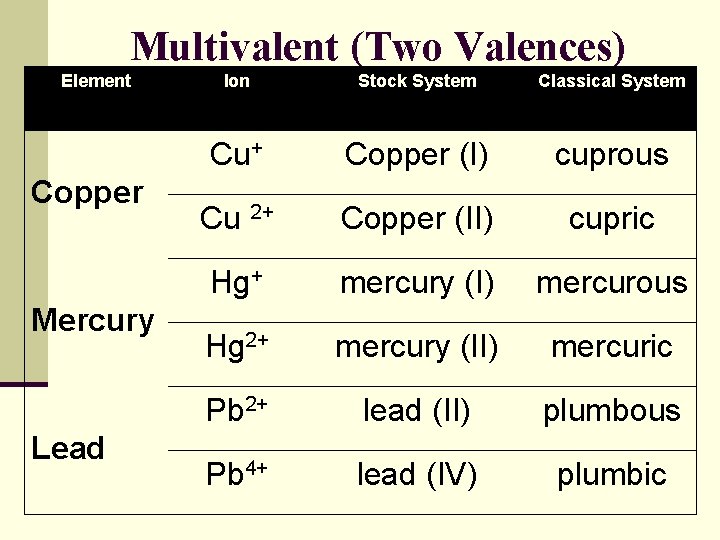
Multivalent (Two Valences) Element Copper Mercury Lead Ion Stock System Classical System Cu+ Copper (I) cuprous Cu 2+ Copper (II) cupric Hg+ mercury (I) mercurous Hg 2+ mercury (II) mercuric Pb 2+ lead (II) plumbous Pb 4+ lead (IV) plumbic

Example of Multivalents n Write the name using the classical system and the stock system for the following: n Hg. O Pb. O 2 n Write the chemical formula from the following names. n Cobaltic iodide stannous fluoride

Polyatomic Compounds n Polyatomic Ions- § compounds that contains 3 elements (often called tertiary compounds).

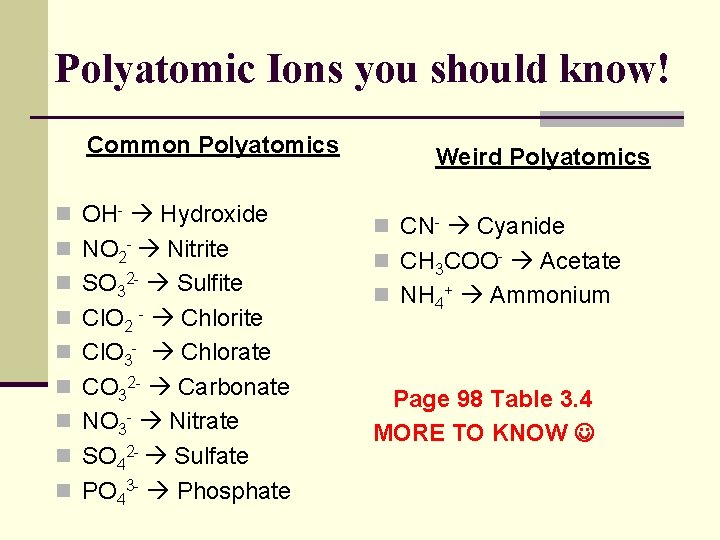
Polyatomic Ions you should know! Common Polyatomics n OH- Hydroxide n NO 2 - Nitrite n SO 32 - Sulfite n Cl. O 2 - Chlorite Weird Polyatomics n CN- Cyanide n CH 3 COO- Acetate n NH 4+ Ammonium n Cl. O 3 - Chlorate n CO 32 - Carbonate n NO 3 - Nitrate n SO 42 - Sulfate n PO 43 - Phosphate Page 98 Table 3. 4 MORE TO KNOW

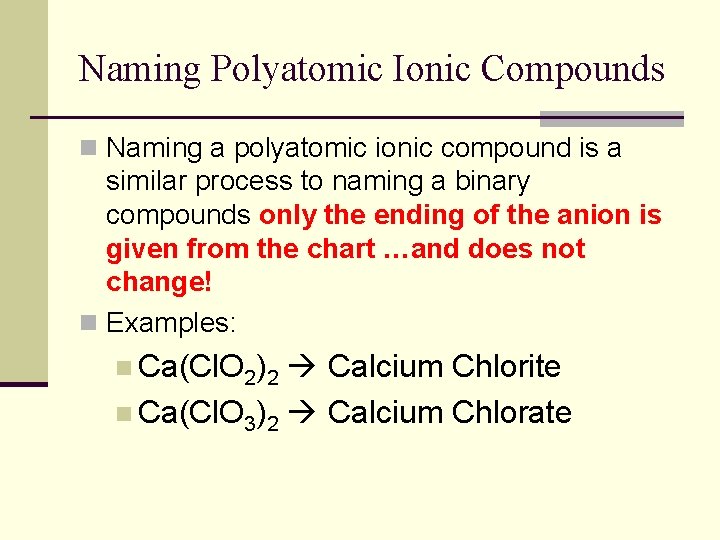
Naming Polyatomic Ionic Compounds n Naming a polyatomic ionic compound is a similar process to naming a binary compounds only the ending of the anion is given from the chart …and does not change! n Examples: n Ca(Cl. O 2)2 Calcium Chlorite n Ca(Cl. O 3)2 Calcium Chlorate

Naming Molecular Compounds

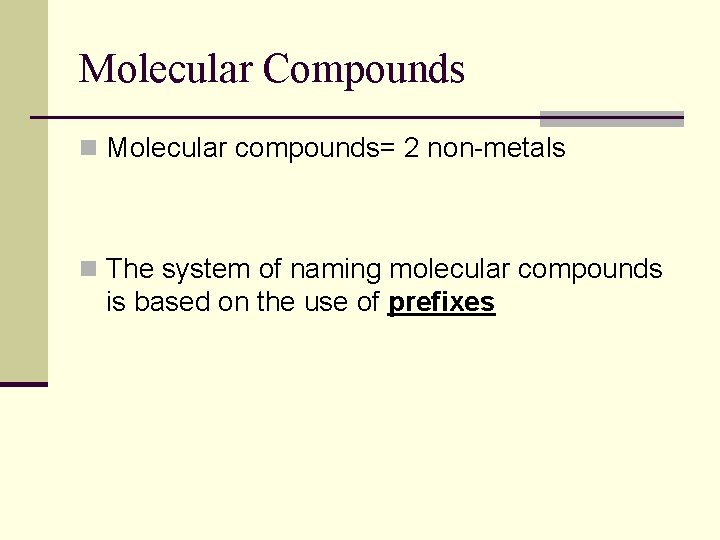
Molecular Compounds n Molecular compounds= 2 non-metals n The system of naming molecular compounds is based on the use of prefixes

Pre-fixes you need to know n 1 Mono- n 6 Hexa- n 2 Di- n 7 Hepta- n 3 Tri- n 8 Octa- n 4 Tetra- n 9 Nona- n 5 Penta- n 10 Deca-

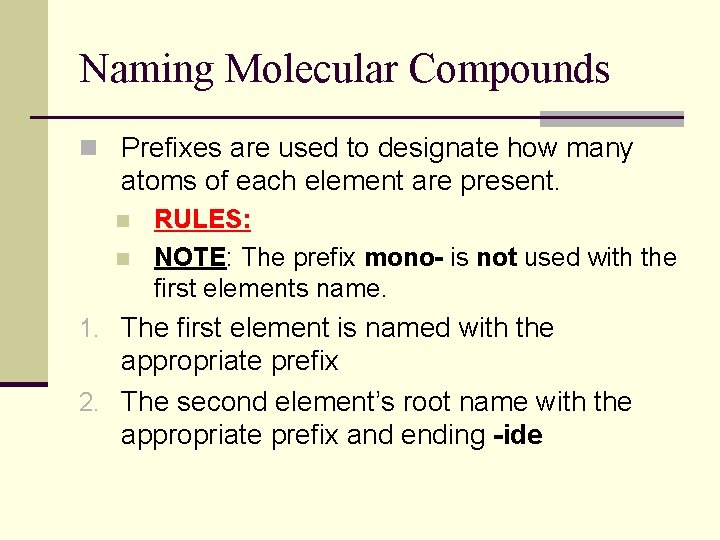
Naming Molecular Compounds n Prefixes are used to designate how many atoms of each element are present. n n RULES: NOTE: The prefix mono- is not used with the first elements name. 1. The first element is named with the appropriate prefix 2. The second element’s root name with the appropriate prefix and ending -ide

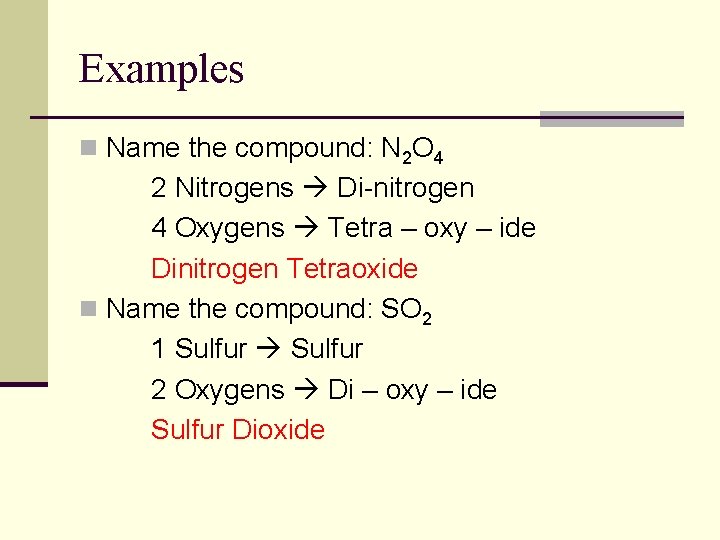
Examples n Name the compound: N 2 O 4 2 Nitrogens Di-nitrogen 4 Oxygens Tetra – oxy – ide Dinitrogen Tetraoxide n Name the compound: SO 2 1 Sulfur 2 Oxygens Di – oxy – ide Sulfur Dioxide

Naming Acids (Chapter 10)

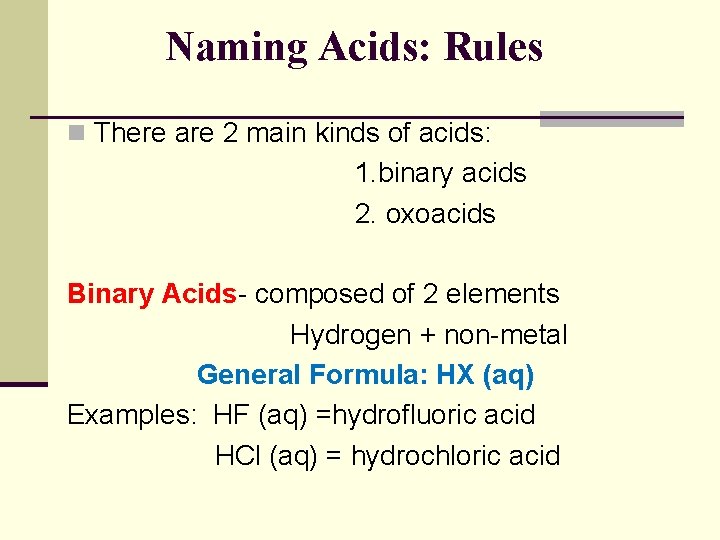
Naming Acids: Rules n There are 2 main kinds of acids: 1. binary acids 2. oxoacids Binary Acids- composed of 2 elements Hydrogen + non-metal General Formula: HX (aq) Examples: HF (aq) =hydrofluoric acid HCl (aq) = hydrochloric acid

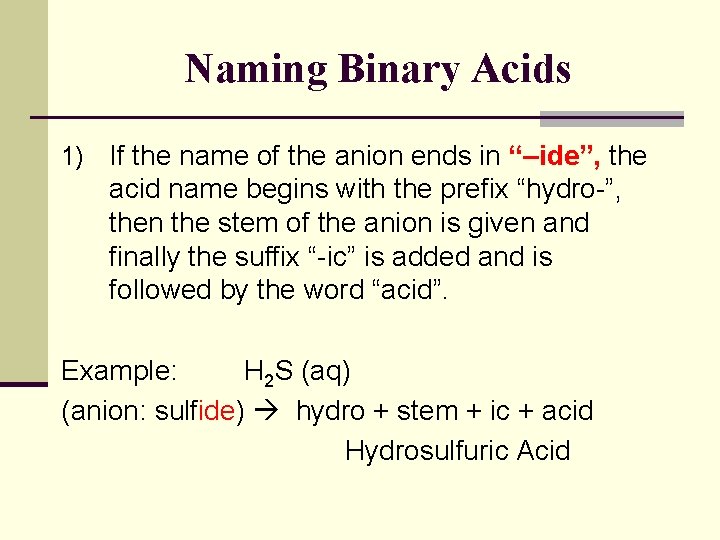
Naming Binary Acids 1) If the name of the anion ends in “–ide”, the acid name begins with the prefix “hydro-”, then the stem of the anion is given and finally the suffix “-ic” is added and is followed by the word “acid”. Example: H 2 S (aq) (anion: sulfide) hydro + stem + ic + acid Hydrosulfuric Acid

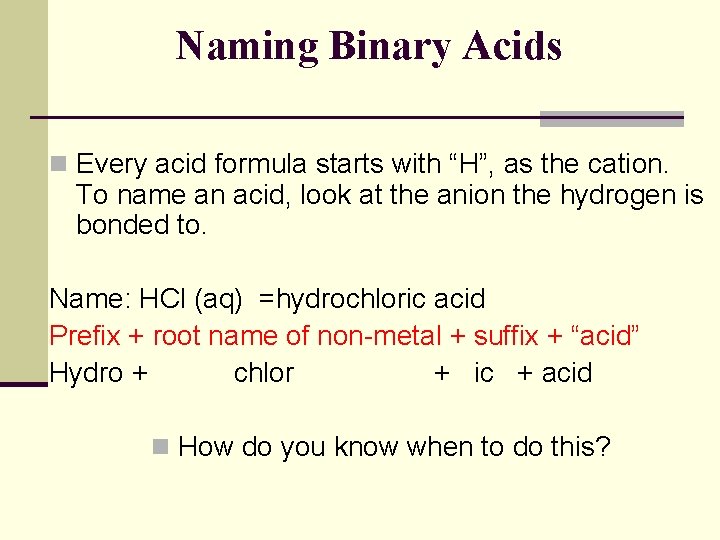
Naming Binary Acids n Every acid formula starts with “H”, as the cation. To name an acid, look at the anion the hydrogen is bonded to. Name: HCl (aq) =hydrochloric acid Prefix + root name of non-metal + suffix + “acid” Hydro + chlor + ic + acid n How do you know when to do this?

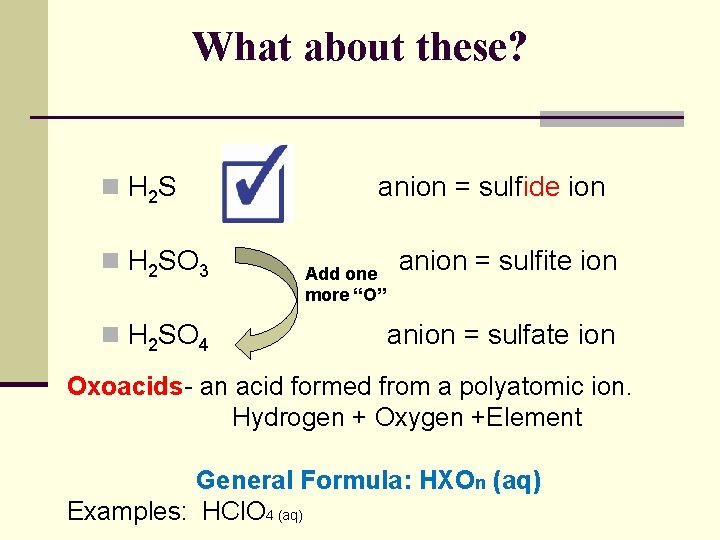
What about these? n H 2 SO 3 n H 2 SO 4 anion = sulfide ion Add one more “O” anion = sulfite ion anion = sulfate ion Oxoacids- an acid formed from a polyatomic ion. Hydrogen + Oxygen +Element General Formula: HXOn (aq) Examples: HCl. O 4 (aq)

Naming Oxoacids “ITE” “OUS” 2) If the name of the anion ends in “–ite”, the acid name is the stem of the anion with the suffix “–ous” and is followed by the word “acid”. H 2 SO 3 (aq) Stop and think about the anion SO 32(anion: sulfite) stem + ous + acid Sulfurous Acid

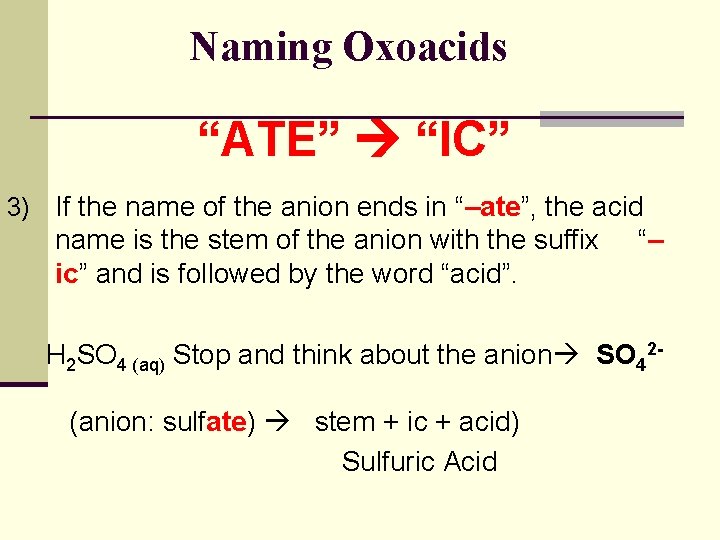
Naming Oxoacids “ATE” “IC” 3) If the name of the anion ends in “–ate”, the acid name is the stem of the anion with the suffix ic” and is followed by the word “acid”. “– H 2 SO 4 (aq) Stop and think about the anion SO 42(anion: sulfate) stem + ic + acid) Sulfuric Acid

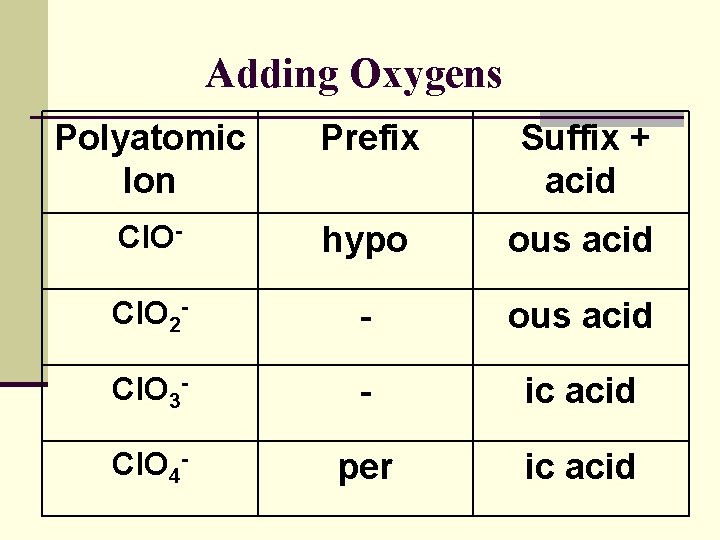
Adding Oxygens Polyatomic Ion Prefix Suffix + acid Cl. O- hypo ous acid Cl. O 2 - - ous acid Cl. O 3 - - ic acid Cl. O 4 - per ic acid

Writing Formulas/Naming Acids n Remember the following statements… “I ate it and it was icky. ” -ate becomes -ic “Rite ous” as (Righteous) -ite becomes -ous

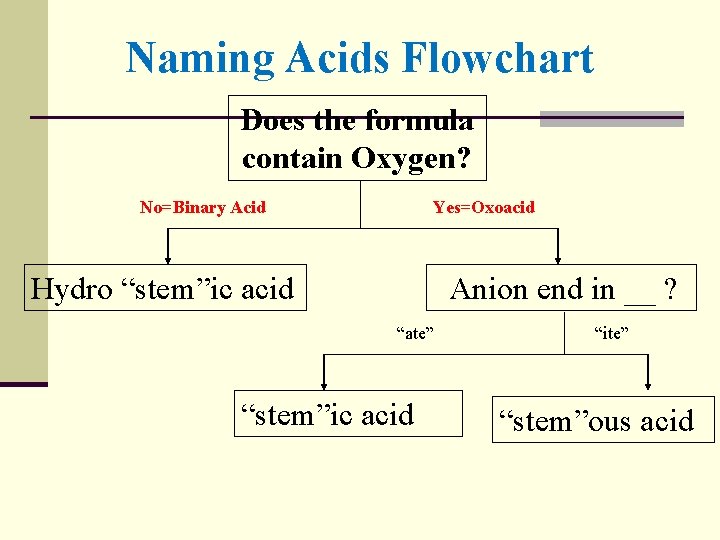
Naming Acids Flowchart Does the formula contain Oxygen? No=Binary Acid Yes=Oxoacid Hydro “stem”ic acid Anion end in __ ? “ate” “stem”ic acid “ite” “stem”ous acid

Writing Formulas for Acids n If the name starts with “hydro” Hydroselenic Acid Write the hydrogen ion with charge. H+1 Write the anion with the proper charge. Se-2 Balance the charges using subscripts. H 2 Se (aq)

Writing Formulas for Acids n If the name contains the suffix “–ous” Nitrous Acid Write the hydrogen ion with charge. H+ Look up the polyatomic ion (nitrite) and write it with the correct charge. NO 2 Balance the charges using subscripts. HNO 2

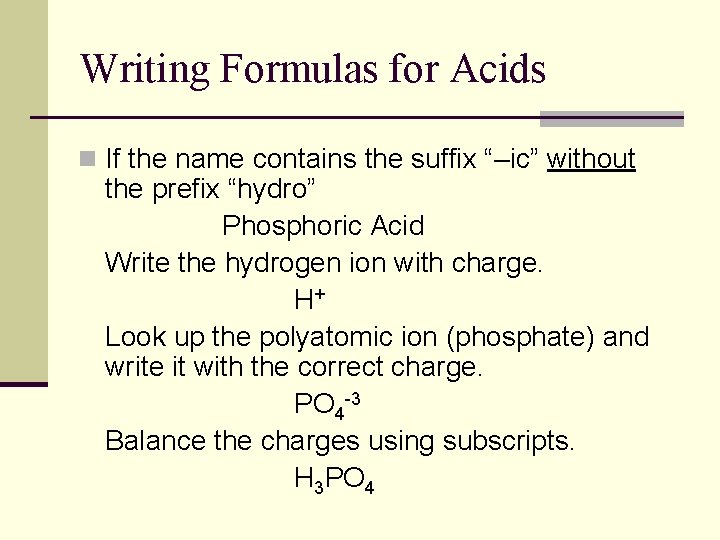
Writing Formulas for Acids n If the name contains the suffix “–ic” without the prefix “hydro” Phosphoric Acid Write the hydrogen ion with charge. H+ Look up the polyatomic ion (phosphate) and write it with the correct charge. PO 4 -3 Balance the charges using subscripts. H 3 PO 4

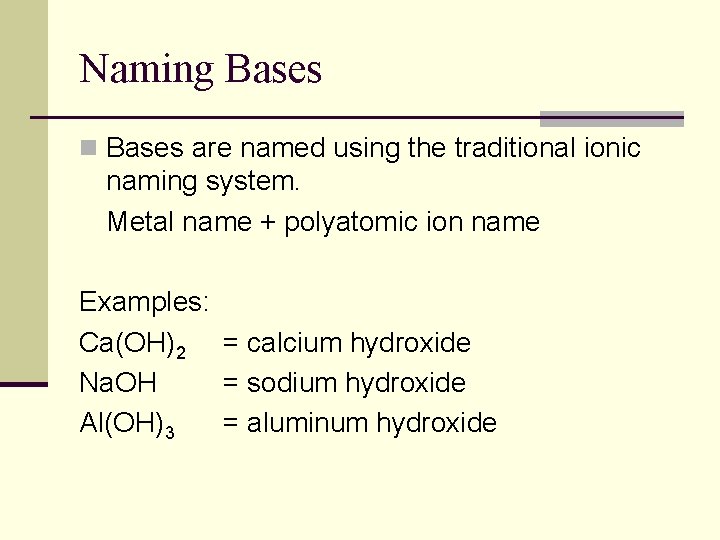
Naming Bases n Bases are named using the traditional ionic naming system. Metal name + polyatomic ion name Examples: Ca(OH)2 = calcium hydroxide Na. OH = sodium hydroxide Al(OH)3 = aluminum hydroxide

Writing Base Formulas n Base formulas are written using the traditional ionic system. Look up the metal ion. Write the symbol with the proper charge. Ca+2 Look up the polyatomic ion. With bases, this will always be hydroxide, OH-1. Balance the charges using subscripts. Ca(OH)2