Chemical Nomenclature Ionic Compounds Ionic Compounds Anion atoms













- Slides: 15

Chemical Nomenclature Ionic Compounds

Ionic Compounds • Anion = atoms with a negative charge • Cation = atoms with a positive charge • Anions and Cations attract one another to form ionic bonds. • Very easy to predict because they always work to neutralize the charges.

Ionic Compounds • To name an ionic compound: • Cation anion –ide • Example: Na. Cl • Sodium Chloride

Practice • Name the following compounds: • Mg. F 2 • Magnesium fluoride • Li. Br • Lithium bromide • Cs 2 O • Cesium oxide • Ca. S • Calcium sulfide

Transition Metals • Transition Metals are tricky because they can take on a variety of charges. • Ex: Cu. Cl 2

Transition Metals • We distinguish between the different forms of transition metals using Roman Numerals. • A Roman Numeral placed within parentheses after the name of a cation denotes the positive charge of that cation.

Keys to Remember • Remember! • Roman Numerals tell the charge of the ions • The charges of the ions determine the ratio in which they combine

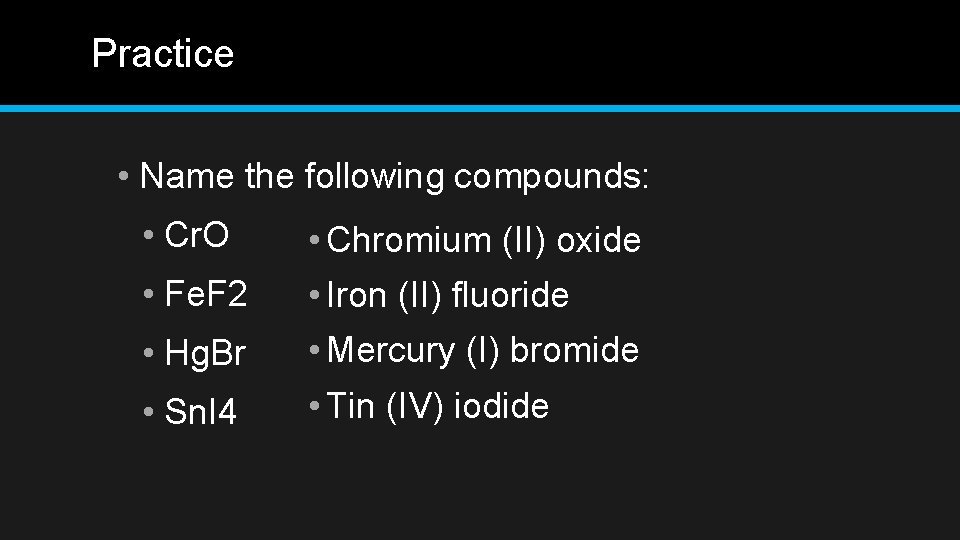
Practice • Name the following compounds: • Cr. O • Chromium (II) oxide • Fe. F 2 • Iron (II) fluoride • Hg. Br • Mercury (I) bromide • Sn. I 4 • Tin (IV) iodide

Practice • Translate the following names into chemical formulas: • Iron (III) oxide • Fe 2 O 3 • Beryllium chloride • Be. Cl 2 • Tin (II) sulfide • Sn. S • Potassium iodide • KI

Polyatomic Ions • Some ions are called ‘polyatomic ions’ because they consist of a group of atoms

Polyatomic Ions • When you write a chemical formula that involves polyatomic ions, you still need to balance charges to form a neutral atom.

Polyatomic Ions • Sometimes polyatomic ions occur multiple times within a compound: 1. Put the polyatomic ion in parentheses 2. add the subscript outside the parentheses • Ex: (SO 4)2

Polyatomic Ions • Ite/ate pairs: –ate ions have one more oxygen than the -ite ion, but the same overall charge.

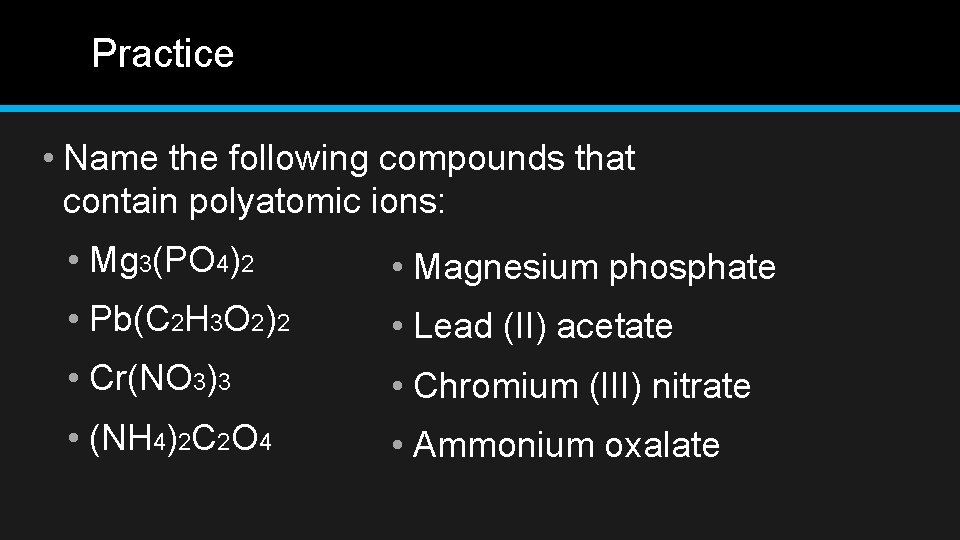
Practice • Name the following compounds that contain polyatomic ions: • Mg 3(PO 4)2 • Magnesium phosphate • Pb(C 2 H 3 O 2)2 • Lead (II) acetate • Cr(NO 3)3 • Chromium (III) nitrate • (NH 4)2 C 2 O 4 • Ammonium oxalate

Practice • Write the formula for the following compounds that contain polyatomic ions: • Potassium sulfate • K 2 SO 4 • Lead (II) dichromate • Pb. Cr 2 O 7 • Ammonium chloride • NH 4 Cl • Sodium hydroxide • Na. OH