Chemical Nomenclature chemistry Ionic Nomenclature For cations Keeps











- Slides: 18

Chemical Nomenclature chemistry

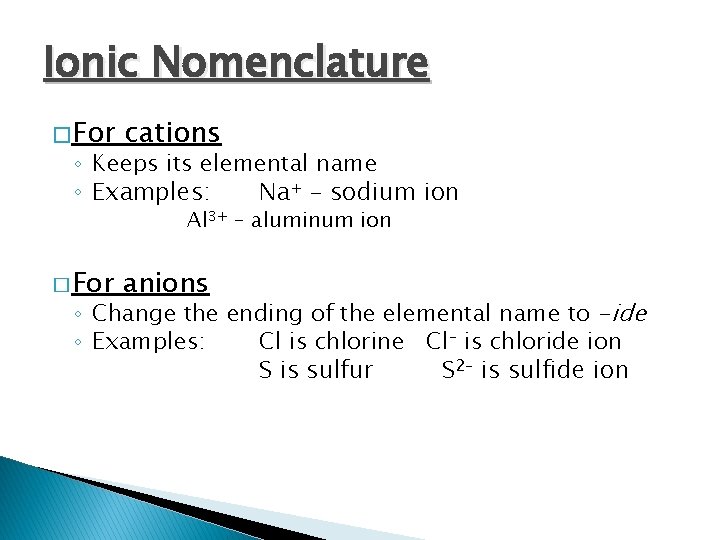
Ionic Nomenclature �For cations ◦ Keeps its elemental name ◦ Examples: Na+ – sodium ion Al 3+ – aluminum ion � For anions ◦ Change the ending of the elemental name to -ide ◦ Examples: Cl is chlorine Cl– is chloride ion S is sulfur S 2– is sulfide ion

Ionic Nomenclature � Metal + Nonmetal � Name Cation first, then anion � Ca. Cl 2 : Calcium chloride � Mg. O: Magnesium oxide

Name the following compound, Ba. I 2. A. B. C. D. Barium iodide Baride iodine Barium iodine Baride iodide

Name this Compound, Ca. Br 2. A. B. C. D. Calcium bromine Carbon bromine Calcium bromide Carbon bromine

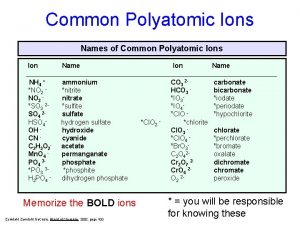
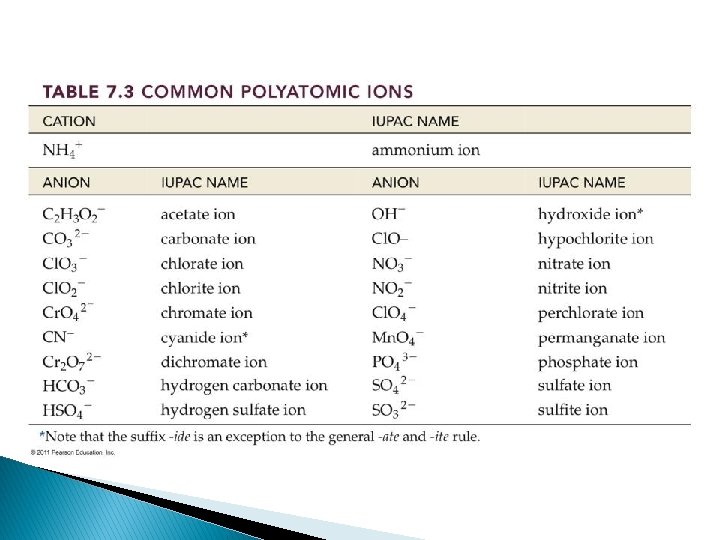
Polyatomic Ions � If Polyatomic is Cation, then list name & add anion name to the end ◦ Ex) (NH 4)Cl: Ammonium chloride � If polyatomic is anion, then name cation and list polyatomic name ◦ Ex) Mg (NO 3)2 Magnesium nitrate

� Look at handout for more info

Name this compound, Na. NO 3 A. B. C. Sodium nitrate Sodium nitrite Sodium nitrogen oxide

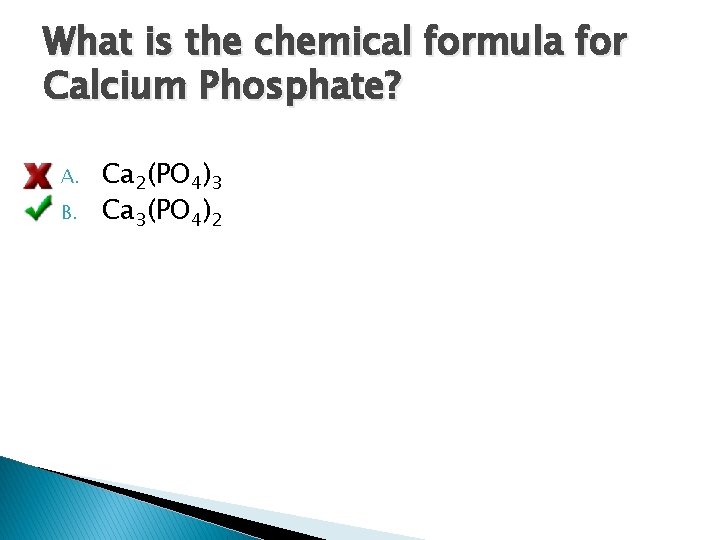
What is the chemical formula for Calcium Phosphate? A. B. Ca 2(PO 4)3 Ca 3(PO 4)2

Name Formula � barium fluoride � aluminum bromide � strontium phosphate

Write the formula for Potassium Nitride

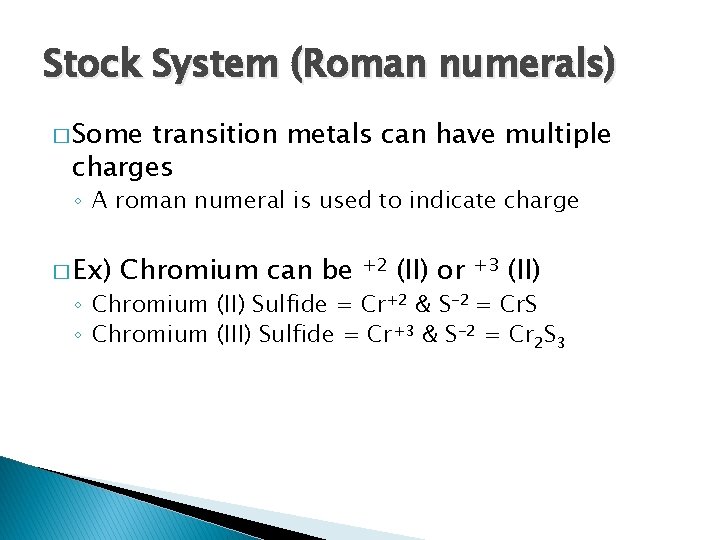
Stock System (Roman numerals) � Some transition metals can have multiple charges ◦ A roman numeral is used to indicate charge � Ex) Chromium can be +2 (II) or +3 (II) ◦ Chromium (II) Sulfide = Cr+2 & S-2 = Cr. S ◦ Chromium (III) Sulfide = Cr+3 & S-2 = Cr 2 S 3

Write the formula for silver (I) hydroxide Name the compound Fe. N Write the formula for Copper (II) Oxide

Molecular (Covalent) Nomenclature for two nonmetals � Prefix System (binary compounds) 1. Least electronegative atom comes first. 2. Add prefixes to indicate # of atoms. Omit mono- prefix on the FIRST element. 3. Mono- is OPTIONAL on the SECOND element 4. Change the ending of the second element to -ide.

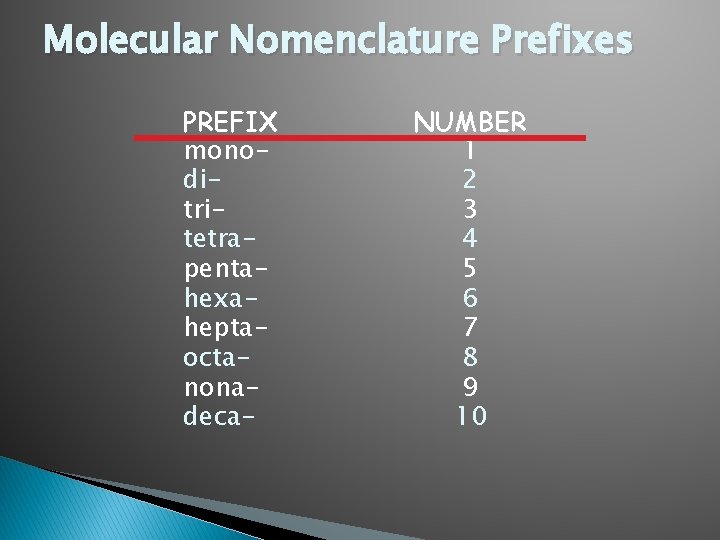
Molecular Nomenclature Prefixes PREFIX monoditritetrapentahexaheptaoctanonadeca- NUMBER 1 2 3 4 5 6 7 8 9 10

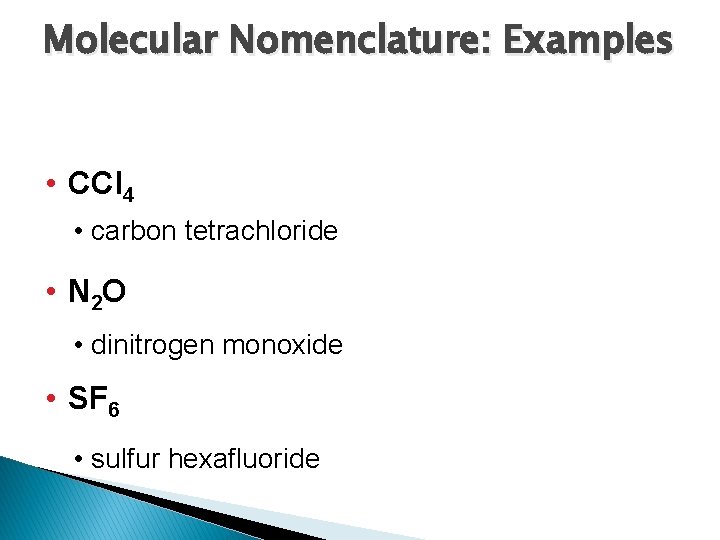
Molecular Nomenclature: Examples • CCl 4 • carbon tetrachloride • N 2 O • dinitrogen monoxide • SF 6 • sulfur hexafluoride

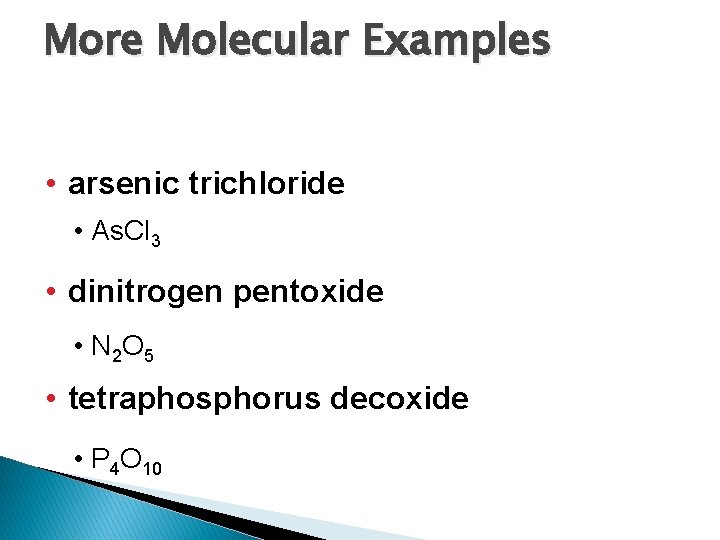
More Molecular Examples • arsenic trichloride • As. Cl 3 • dinitrogen pentoxide • N 2 O 5 • tetraphosphorus decoxide • P 4 O 10

Name the compound CCl 4 What is the chemical formula for Sulfur Trioxide
 Cations with multiple charges
Cations with multiple charges Ionic binary compounds multiple charge cations
Ionic binary compounds multiple charge cations Scientific taxonomy
Scientific taxonomy Nomenclature of binary ionic compounds
Nomenclature of binary ionic compounds Ionic nomenclature
Ionic nomenclature Cell chapter 20
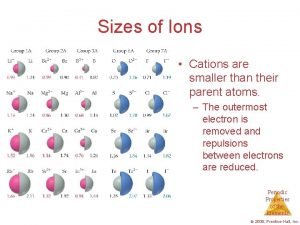
Cell chapter 20 Why are cations smaller
Why are cations smaller No21- ion name
No21- ion name Common monatomic ions
Common monatomic ions Three ionic compounds
Three ionic compounds Which is an example of a polyatomic ion?h2co3-mg+ne+ -
Which is an example of a polyatomic ion?h2co3-mg+ne+ - Schematic diagram of group 2 cations
Schematic diagram of group 2 cations Common ions
Common ions Paper chromatography separation of cations and dyes
Paper chromatography separation of cations and dyes Match the cation to its color.
Match the cation to its color. Pb(no3)2 + ki
Pb(no3)2 + ki Nomenclature of organic compounds
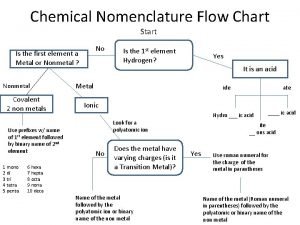
Nomenclature of organic compounds Chemical nomenclature flow chart
Chemical nomenclature flow chart Alkene alcohol naming
Alkene alcohol naming