Chemical Names Formulas Ionic Compounds IV ION 1









- Slides: 11

Chemical Names & Formulas Ionic Compounds IV

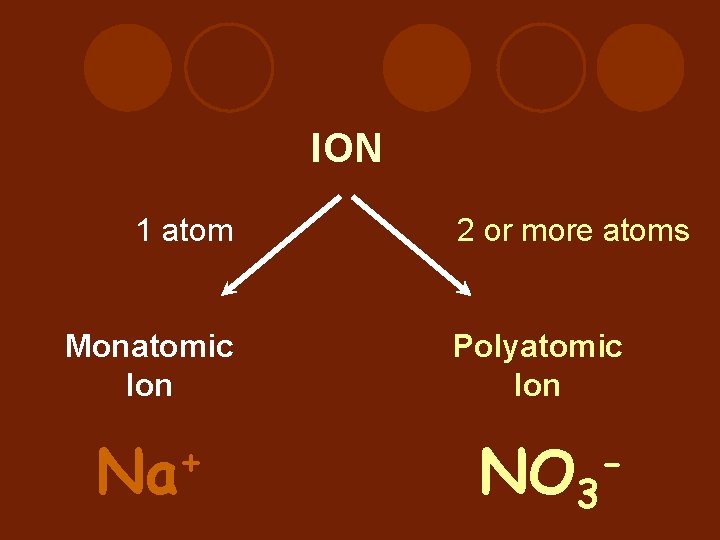
ION 1 atom 2 or more atoms Monatomic Ion Polyatomic Ion + Na NO 3 -

Monatomic Cations l metals in Groups 1 A, 2 A, and 3 A form cations with positive charges equal to their group number l names of the cations (metals) are the same as the name of the metal, followed by the word ion l Example: Beryllium forms Be 2+ → beryllium ion

Transition Metals l Many transition metals form more than one cation. l A Roman numeral in parentheses is placed after the name of the element to indicate the numerical value of the charge. l Example: tin forms Sn 2+ → tin(II) ion

Monatomic Anions • The charge of any ion of a Group A nonmetal is determined by subtracting 8 from the group number. • Anion (nonmetals) names start with the stem of the element name and end in ide. • Example: sulfur forms S 2 - → sulfide

Writing Ionic Compound Formulas l Write the cation symbol first, anion last. l Overall charge must equal zero. ¡Use criss-cross method to balance charges. ¡Formulas are written in lowest whole number ratio. ¡If charges cancel, just write symbols. l Use parentheses to show more than one polyatomic ion.

Writing Ionic Formulas Practice l potassium chloride ¡K+ Cl- KCl l magnesium nitrate ¡Mg 2+ NO 3 - Mg(NO 3)2 l copper(II) chloride ¡Cu 2+ Cl- Cu. Cl 2

Writing Ionic Formulas Practice Write the formulas for the following ionic compounds: Na 2 S l Sodium sulfide = l Lead(II) phosphate = Pb 3(PO 4)2 l Magnesium carbonate = Mg. CO 3

Naming Ionic Compounds Ionic Names l Write the name of the metallic cation first. l Write the name of the nonmetallic anion second (ending in -ide. ) l Polyatomic ions have special names. l Use Roman numerals to show the ion’s charge if more than one is possible.

Naming Ionic Compounds Practice l Na. Br ¡sodium bromide l Na 2 CO 3 ¡sodium carbonate l Fe. Cl 3 ¡iron(III) chloride

Naming Ionic Compounds Practice Name the following ionic compounds: l Fe. Br 3 = Iron(III) bromide l KOH = l Na 2 Cr 2 O 7 Potassium hydroxide = Sodium dichromate