Chemical Names Formulas CHAPTER 7 1 Chemical Names












- Slides: 16

Chemical Names & Formulas CHAPTER 7. 1

Chemical Names & Formulas ØCommon chemical names give no information about chemical composition. ◦ Example: vinegar, baking soda, water. ØChemists use systematic methods for naming compounds and writing chemical formulas. ◦ Example: HC 2 H 3 O 2, Na. HCO 3, H 2 O

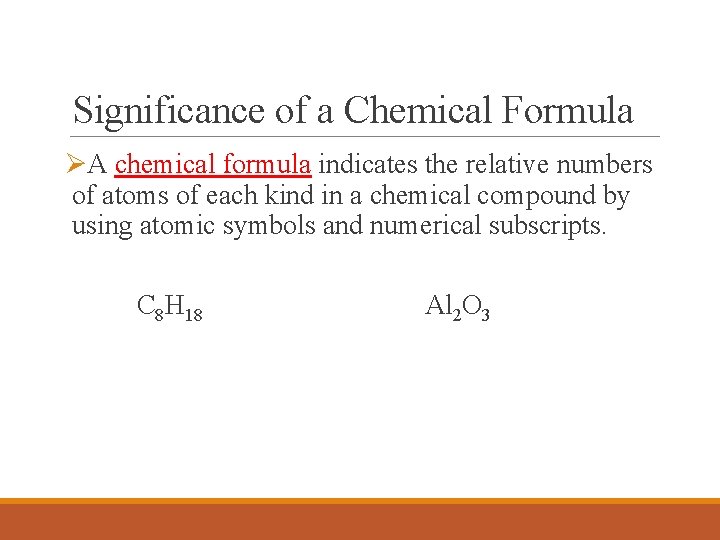
Significance of a Chemical Formula ØA chemical formula indicates the relative numbers of atoms of each kind in a chemical compound by using atomic symbols and numerical subscripts. C 8 H 18 Al 2 O 3

Monatomic Ions ØIons formed from a single atom are known as monatomic ions. ØNaming monatomic ions: ◦ Cations are given the element’s name. ◦ Example: Ca 2+: calcium; Na+: sodium; Al 3+: aluminum ◦ Anions have a slightly different name than parent atom; the ending of the element name is dropped and replaced with -ide. ◦ Example: Cl-: chloride; O 2 -: oxide; N 3 -: nitride; I-: iodide

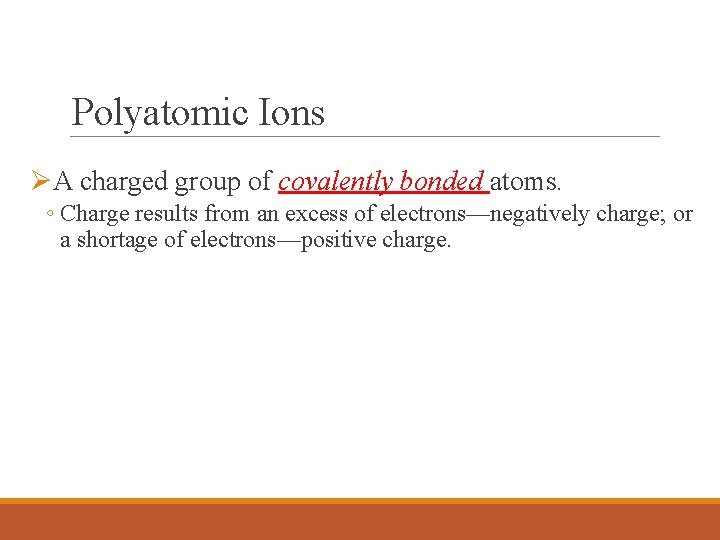
Polyatomic Ions ØA charged group of covalently bonded atoms. ◦ Charge results from an excess of electrons—negatively charge; or a shortage of electrons—positive charge.

Nomenclature of Ionic Compounds The nomenclature, or naming system, of binary ionic compounds involves combining the names of the compound’s positive and negative ions.

Naming Ionic Compounds The cation is always named first and the anion second. ◦ Cation always has the name of its parent element. ◦ Anion is named by taking the root of the element name and adding –ide. Examples: 1. Na. Cl 2. Ca. Br 2 3. Sr. F 2 4. Ba. O

Naming Ionic Compounds with Transition Metals and Grp 13 -16 Metals ØThe cation of a transition metal (or ‘other metals’) is always named first followed by roman numeral, then the name of the anion. ◦ All transition metal cations, except Zn 2+, Cd 2+ and Ag+ (which always have these charges), must show oxidation number (charge) in parenthesis using roman numerals. ◦ For cations in groups 13 -16, except Al, include parenthesis after name to show oxidation number (charge).

Naming Ionic Compounds with Transition Metals and Grp 13 -16 Metals Examples: 1. Cu. Cl 2 2. Fe 2 O 3 3. Cr. F 3 4. Co. Cl 3 5. Fe. S 6. Pb. Cl 2

Name the following compounds: 1. Ag 2 O 2. Ca(OH)2 3. KCl. O 3 4. NH 4 OH 5. Fe 2(Cr. O 4)3 6. KCl. O 7. Na. I 8. Ca(NO 2)2

Writing Chemical Formulas for Ionic Compounds 1. Write the symbols for the ions side by side. 2. Write chemical formula using subscripts to indicate the number of atoms of each elements present in the compound. ◦ The charges of the ions will tell you how many atoms of each element is needed to form a neutral compound ◦ Cation is always first, then anion.

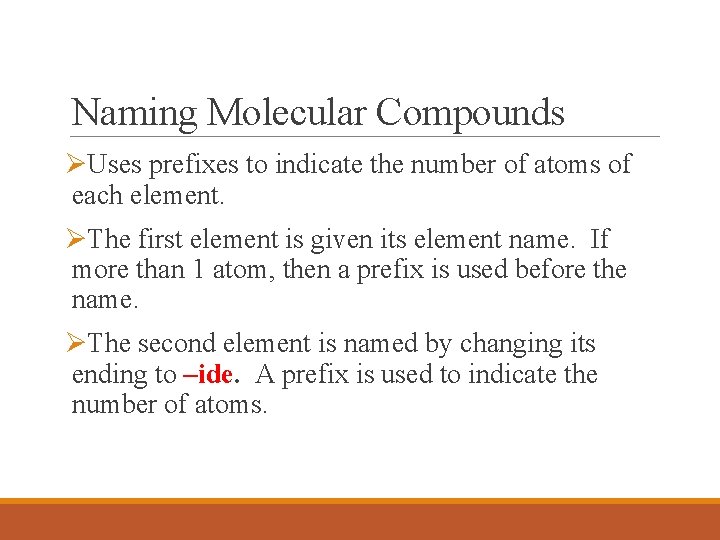
Naming Molecular Compounds ØUses prefixes to indicate the number of atoms of each element. ØThe first element is given its element name. If more than 1 atom, then a prefix is used before the name. ØThe second element is named by changing its ending to –ide. A prefix is used to indicate the number of atoms.

Numerical Prefixes 1 mono- 2 di- 3 tri- 4 tetra- 5 penta- 6 hexa- 7 hepta- 8 octa- 9 nona- 10 deca-

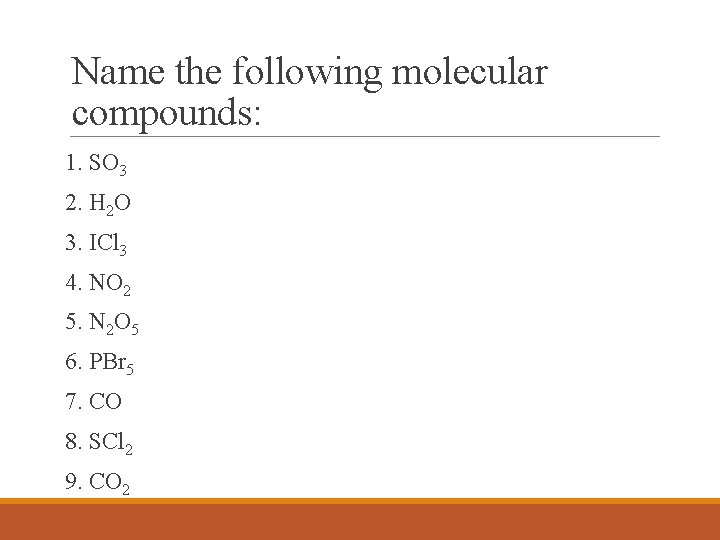
Name the following molecular compounds: 1. SO 3 2. H 2 O 3. ICl 3 4. NO 2 5. N 2 O 5 6. PBr 5 7. CO 8. SCl 2 9. CO 2

Write the chemical formula for the following covalent compounds: 1. Carbon monoxide 2. Carbon tetrachloride 3. Diarsenic pentoxide 4. Silicon dioxide 5. Phosphorus pentafluoride 6. Sulfur dioxide 7. Nitrogen trihydride 8. Dinitrogen tetroxide

Naming Acids An acid is a distinct type of molecular compound. It is a solution in water (aqueous solution). (Table 5 pg. 230)
 Representative metal
Representative metal Chemical names and formulas worksheet chapter 9
Chemical names and formulas worksheet chapter 9 Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas A chemical formula shows
A chemical formula shows Empirical formula pogil
Empirical formula pogil Modern chemistry chapter 7 test
Modern chemistry chapter 7 test Ag3po4compound name
Ag3po4compound name Chapter 6 section 3 compound names and formulas answer key
Chapter 6 section 3 compound names and formulas answer key Writing formulas from names
Writing formulas from names Section 3 names and formulas for ionic compounds
Section 3 names and formulas for ionic compounds Section 3 names and formulas for ionic compounds
Section 3 names and formulas for ionic compounds Writing and naming chemical formulas
Writing and naming chemical formulas Counting atoms worksheet
Counting atoms worksheet Criss cross method for ionic compounds
Criss cross method for ionic compounds Mole concept formulae
Mole concept formulae Superscript
Superscript Covalent covalent bond
Covalent covalent bond