CHEMICAL NAMES AND FORMULAS Nomenclature Naming Monatomic Ions








- Slides: 22

CHEMICAL NAMES AND FORMULAS Nomenclature

Naming Monatomic Ions • Monatomic ions – single atom with a (+) or (-) charge • Cations • Group 1 A, 2 A, and 3 A elements • Name is the same as the metal followed by the word ion or cation • examples • Na+ - sodium ion or sodium cation • Ca 2+ - calcium ion or calcium cation

Naming Monatomic Ions • Anions • Groups 5 A, 6 A, & 7 A • The name is not the same as the element’s name < different from the rules for naming cations> • Start with the stem of the element name and end in –ide. • Examples • fluorine (F-) fluoride ion • oxygen (O-2) oxide ion • Nitrogen (N-3) nitride ion • Group 4 A and 8 A elements do not usually form ions • Now it’s your turn! Do the problems corresponding to the naming monatomic ions heading.

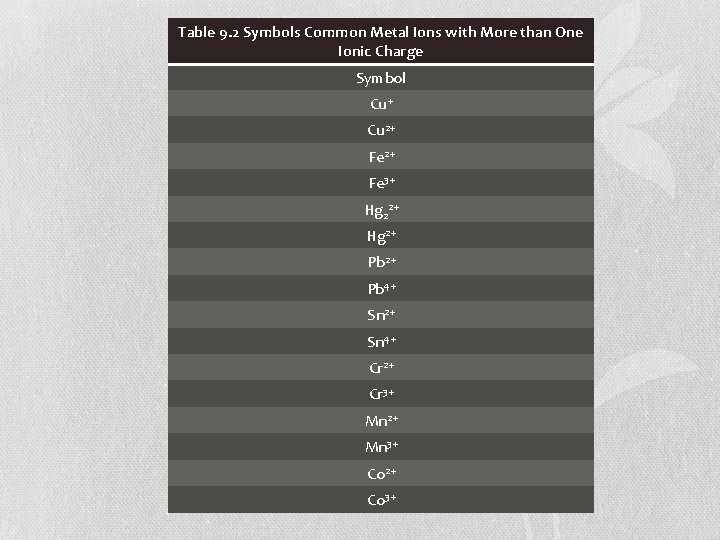
Ions of Transition Metals • The charges of the cations of many transition metals must be determined by the number of electrons lost • Some transition metals can more than one ion with different charges.

Table 9. 2 Symbols Common Metal Ions with More than One Ionic Charge Symbol Cu+ Cu 2+ Fe 3+ Hg 22+ Hg 2+ Pb 4+ Sn 2+ Sn 4+ Cr 2+ Cr 3+ Mn 2+ Mn 3+ Co 2+ Co 3+

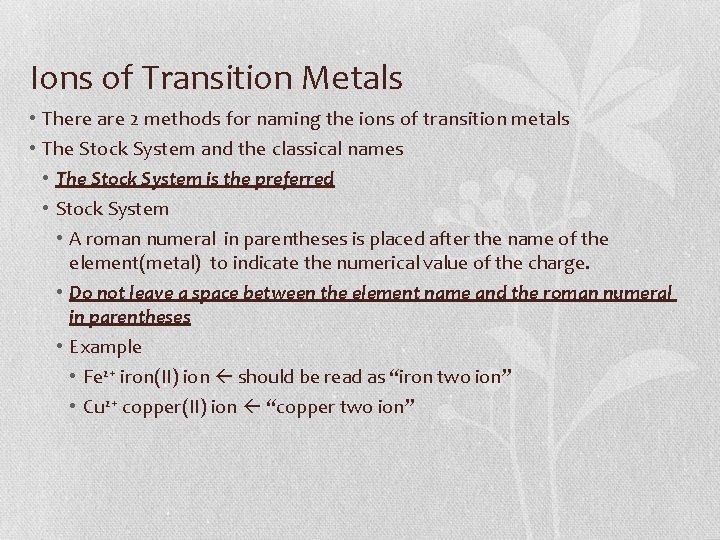
Ions of Transition Metals • There are 2 methods for naming the ions of transition metals • The Stock System and the classical names • The Stock System is the preferred • Stock System • A roman numeral in parentheses is placed after the name of the element(metal) to indicate the numerical value of the charge. • Do not leave a space between the element name and the roman numeral in parentheses • Example • Fe 2+ iron(II) ion should be read as “iron two ion” • Cu 2+ copper(II) ion “copper two ion”

Ions of Transition Metals • Classical Names not the preferred method, however you must know it. • Uses a root word with different suffixes at the end of the word • The suffix –ous is used to name the cation with the lower of the two ionic charges • The suffix –ic is used to name the cation with the higher of the two ionic charges. Examples ferrum is latin for iron therefore ferr- is the root of the word • Fe 2+ - ferrous ion • Fe 3+ - ferric ion stannum is latin for tin therefore stann- is the root of the word • Sn 2+ - stannous ion • Sn 4+ - stannic ion

Ions of Transition Metals • Classical Names • Disadvantages • Does not tell you the actual charge • Only tells you whether the cation has the smaller or larger of the charges • A few transition metals have only one charge • Do not use roman numerals when using the stock system • Some of these exceptions are • silver - Ag+, cadmium – Cd 2+, & zinc 2+ Complete the section also labeled Ions of Transition Metals Now it’s your turn!

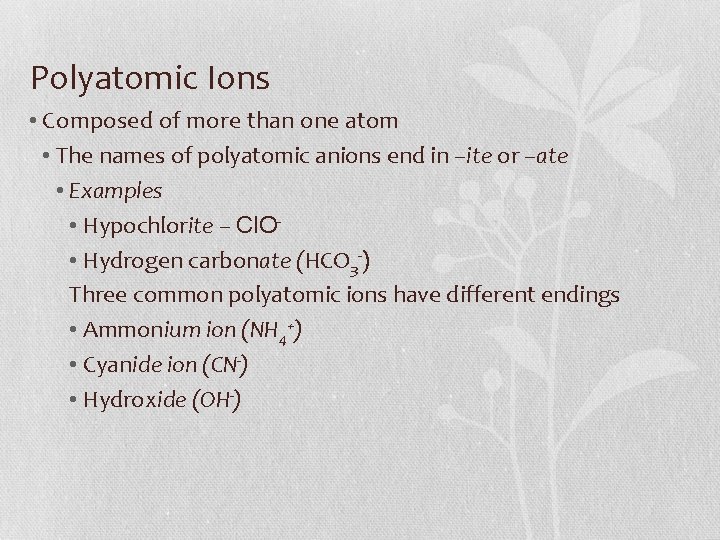
Polyatomic Ions • Composed of more than one atom • The names of polyatomic anions end in –ite or –ate • Examples • Hypochlorite – Cl. O • Hydrogen carbonate (HCO 3 -) Three common polyatomic ions have different endings • Ammonium ion (NH 4+) • Cyanide ion (CN-) • Hydroxide (OH-)

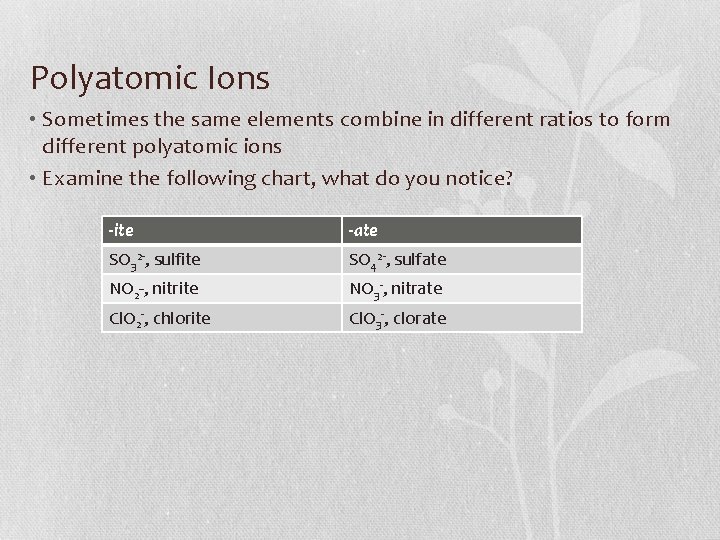
Polyatomic Ions • Sometimes the same elements combine in different ratios to form different polyatomic ions • Examine the following chart, what do you notice? -ite -ate SO 32 -, sulfite SO 42 -, sulfate NO 2 -, nitrite NO 3 -, nitrate Cl. O 2 -, chlorite Cl. O 3 -, clorate

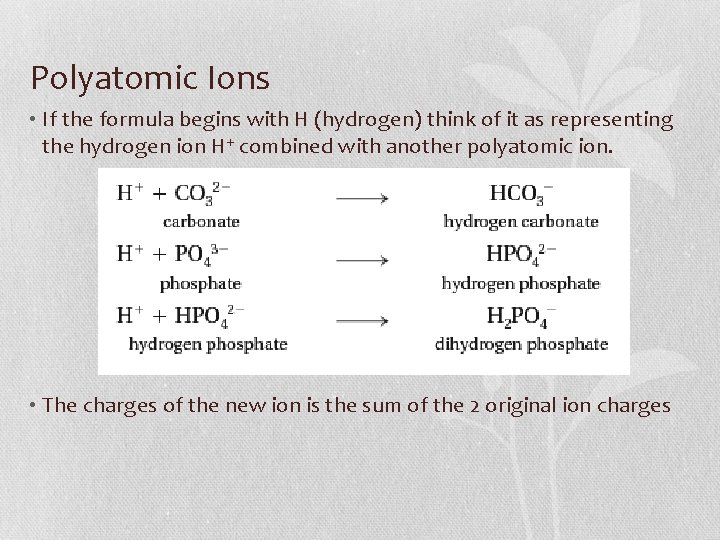
Polyatomic Ions • If the formula begins with H (hydrogen) think of it as representing the hydrogen ion H+ combined with another polyatomic ion. • The charges of the new ion is the sum of the 2 original ion charges

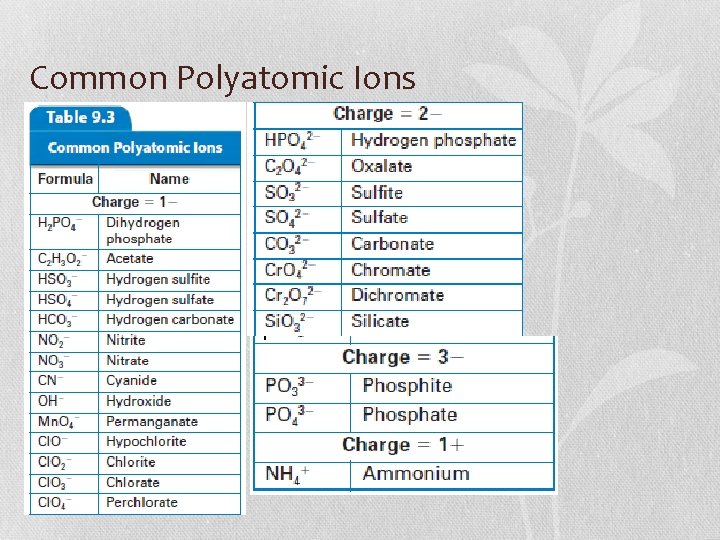
Common Polyatomic Ions

Naming and Writing Formulas for Ionic Compounds

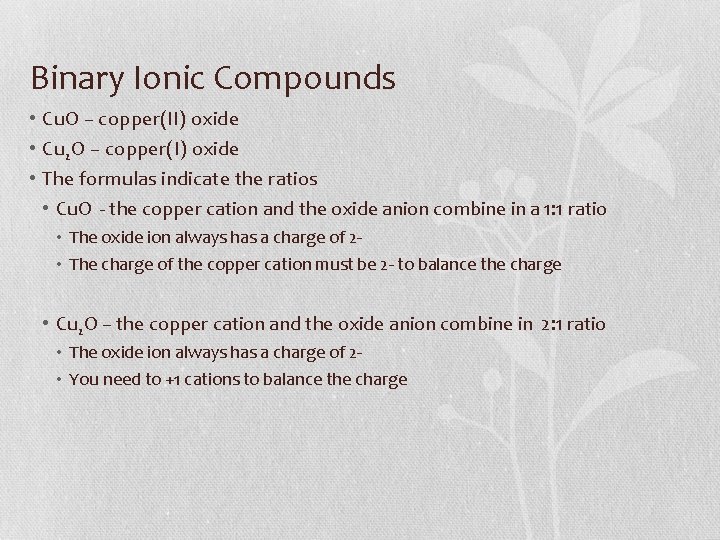
Binary Ionic Compounds • Antoine-Laurent Lavoisier worked to identify the composition of many compounds • Worked with other chemists to develop a system for naming compounds – the chemical names today are based on their work • Binary compound – composed of two elements and can be ionic or molecular • If you know the formula of an ionic compound, it is easy to write the name • Place the cation name first, followed by the anion name

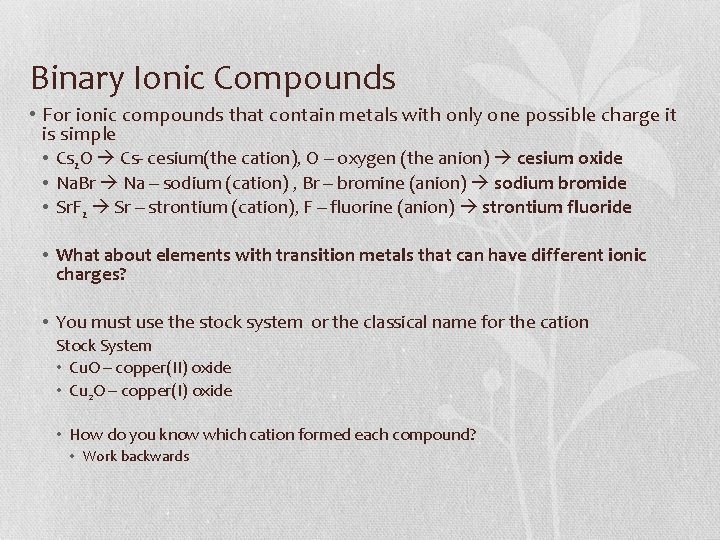
Binary Ionic Compounds • For ionic compounds that contain metals with only one possible charge it is simple • Cs 2 O Cs- cesium(the cation), O – oxygen (the anion) cesium oxide • Na. Br Na – sodium (cation) , Br – bromine (anion) sodium bromide • Sr. F 2 Sr – strontium (cation), F – fluorine (anion) strontium fluoride • What about elements with transition metals that can have different ionic charges? • You must use the stock system or the classical name for the cation Stock System • Cu. O – copper(II) oxide • Cu 2 O – copper(I) oxide • How do you know which cation formed each compound? • Work backwards

Binary Ionic Compounds • Cu. O – copper(II) oxide • Cu 2 O – copper(I) oxide • The formulas indicate the ratios • Cu. O - the copper cation and the oxide anion combine in a 1: 1 ratio • The oxide ion always has a charge of 2 • The charge of the copper cation must be 2 - to balance the charge • Cu 2 O – the copper cation and the oxide anion combine in 2: 1 ratio • The oxide ion always has a charge of 2 • You need to +1 cations to balance the charge

Binary Ionic Compounds • Using the classical names for the ions • Sn. F 2 and Sn. S 2 • The fluoride anion has a charge of -1 and the sulfur anion has a charge of -2 • Sn. F 2 • The ratio of Sn to F is 1: 2 therefore the charge of Sn must be +2 to balance the -2 charge of the two fluoride anions • stannous fluoride because +2 is the lower of the ionic charges for tin • Sn. S 2 • The ratio of Sn to S is 1: 2, S is a -2 so to balance the -4 charge of S anions the charge of tin must be +4 • Stannic sulfide because +4 is the higher of the ionic charges for tin

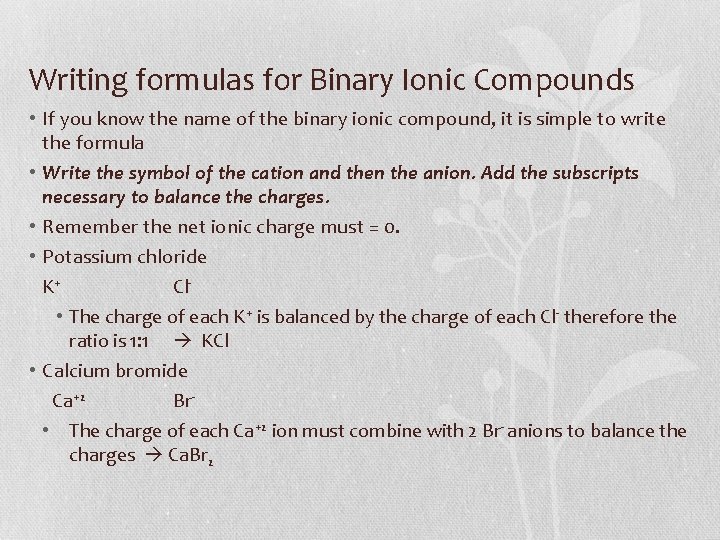
Writing formulas for Binary Ionic Compounds • If you know the name of the binary ionic compound, it is simple to write the formula • Write the symbol of the cation and then the anion. Add the subscripts necessary to balance the charges. • Remember the net ionic charge must = 0. • Potassium chloride K+ Cl • The charge of each K+ is balanced by the charge of each Cl- therefore the ratio is 1: 1 KCl • Calcium bromide Ca+2 Br • The charge of each Ca+2 ion must combine with 2 Br- anions to balance the charges Ca. Br 2

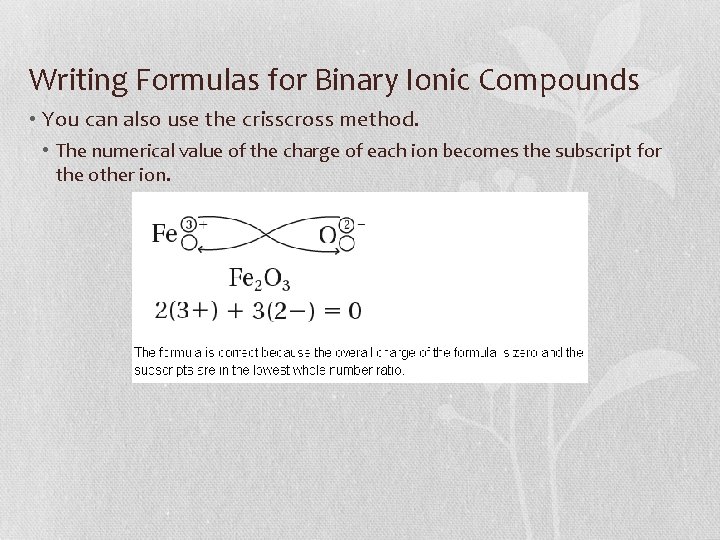
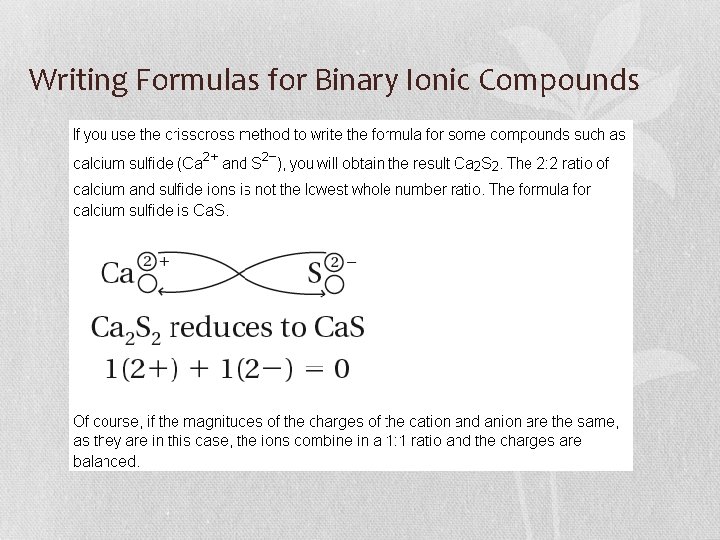
Writing Formulas for Binary Ionic Compounds • You can also use the crisscross method. • The numerical value of the charge of each ion becomes the subscript for the other ion.

Writing Formulas for Binary Ionic Compounds

Compounds with Polyatomic Ions • -ate or –ite ending indicates a compound with a polyatomic ion that includes oxygen • Writing the chemical formula requires the same process. • Write the symbols and then add the subscripts necessary to balance the charge Examples • Calcium carbonate Ca+2 CO 32 - 1: 1 ratio Ca. CO 3 • Calcium nitrate Ca+2 NO 3 - 1: 2 ratio Ca(NO 3)2 Use parentheses around the polyatomic ion when more than one is required to balance the charges and place the subscript after the parentheses. The criss-cross method can also be used

Naming Compounds with Polyatomic Ions • When given the formula • 1 st – recognize that the compound contains a polyatomic ion • Yes it will be necessary for you to memorize the common polyatomic ions!! • 2 nd – state the cation first and then the anion Examples Li. CN – lithium cyanide Na. Cl. O – sodium hypochlorite (Cl. O-) this is a polyatomic ion with the name hypochlorite because it has one less oxygen than Cl. O 2 - which is has the name chlorite.