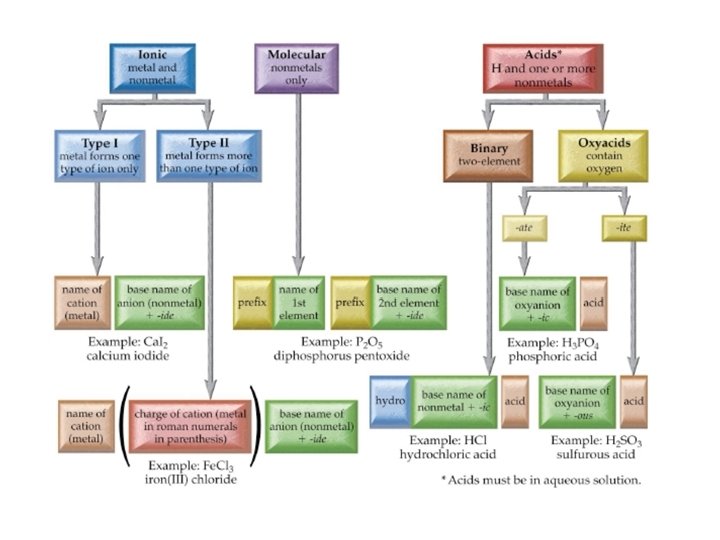
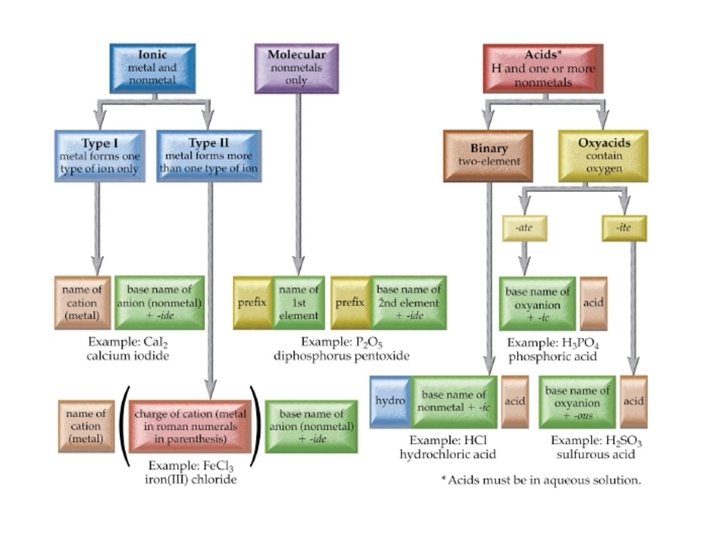
Chemical Names and Formulas Monatomic Ions Cations Groups




- Slides: 22

Chemical Names and Formulas

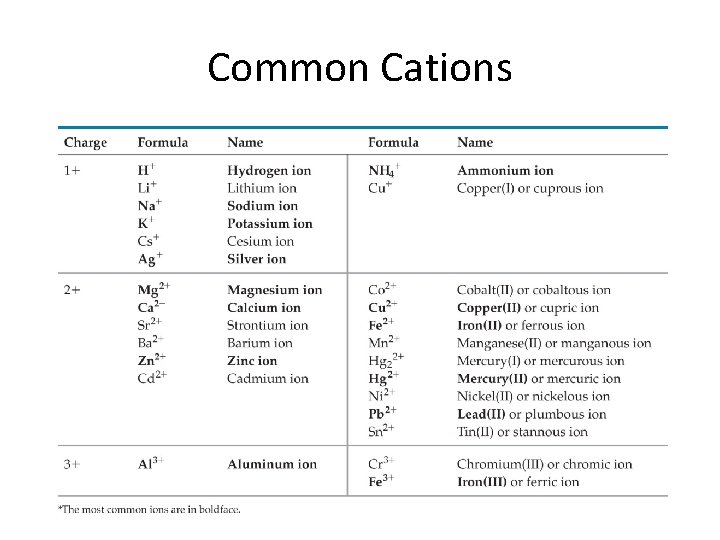
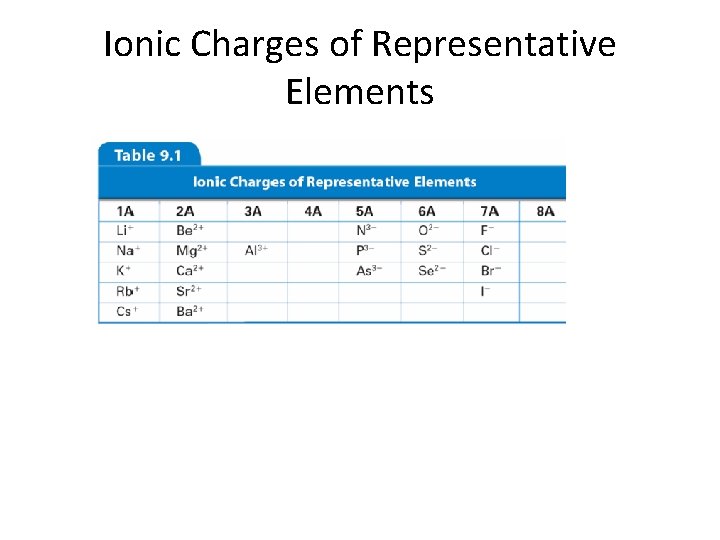
Monatomic Ions Cations • Groups 1 A, 2 A, 3 A charges = group number • Name: element name “ion” • Examples: • Na+ • Mg 2+ • Al 3+ sodium ion magnesium ion aluminum ion

Common Cations

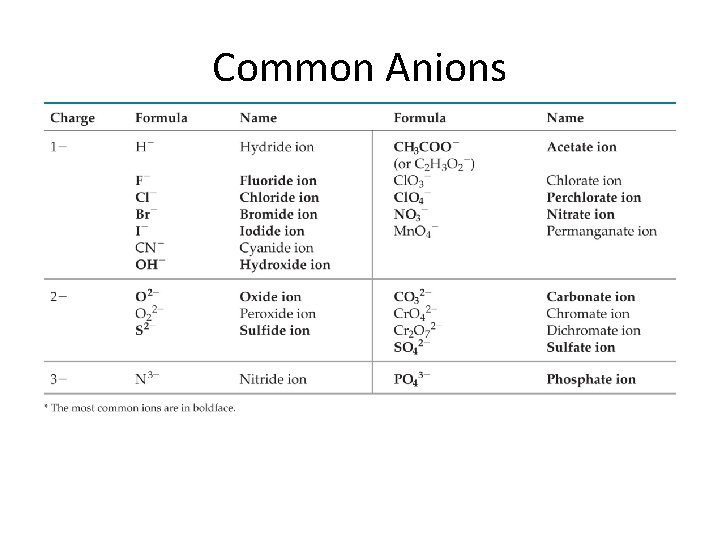
Monatomic Ions Anions • Group A element charges = 8 - group number • Name: stem of element name + ide • Examples – Cl– S 2– O 2– N 3 - chloride sulfide oxide nitride

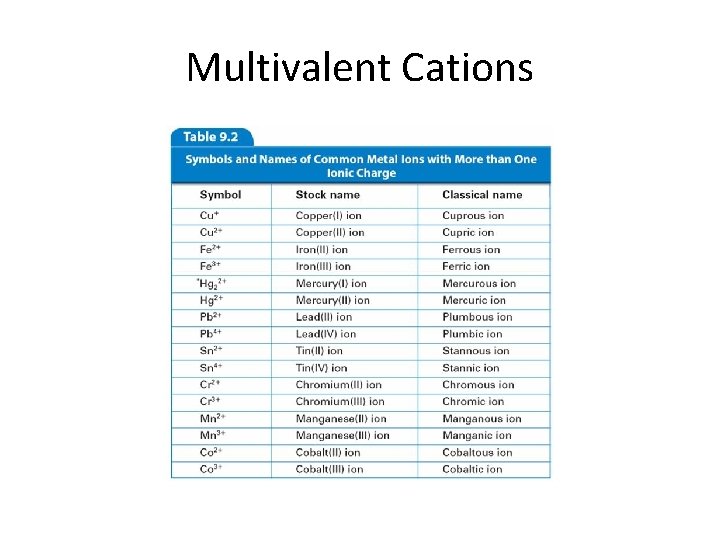
Monatomic Ions Transition Metals (Group B) • Charges can’t be predicted from Periodic Table • Some are “multivalent” – Form more than one ion • Name: element name (charge) “ion” • “Stock” naming system – Use Roman numerals to indicate charge • • • Examples: Fe 2+ Fe 3+ Cu 2+ Stock name iron (II) ion iron (III) ion copper (II) ion Classical name ferrous ion ferric ion cuprous ion cupric ion

Ionic Charges of Representative Elements

Multivalent Cations

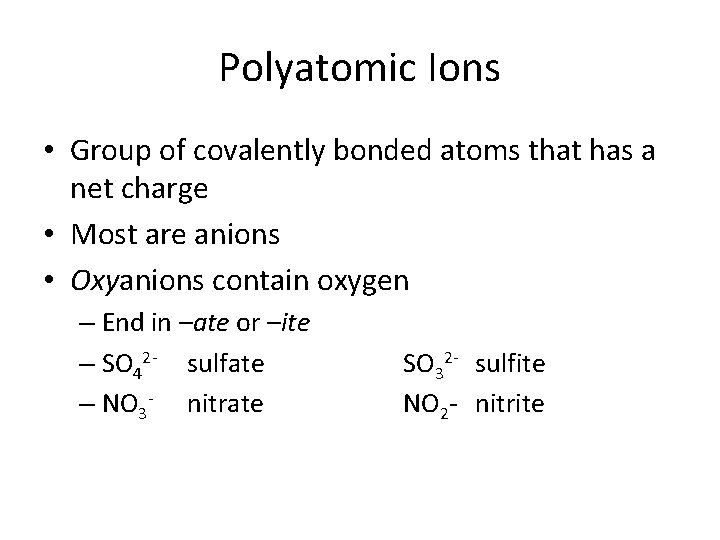
Polyatomic Ions • Group of covalently bonded atoms that has a net charge • Most are anions • Oxyanions contain oxygen – End in –ate or –ite – SO 42 - sulfate – NO 3 - nitrate SO 32 - sulfite NO 2 - nitrite

Common Anions

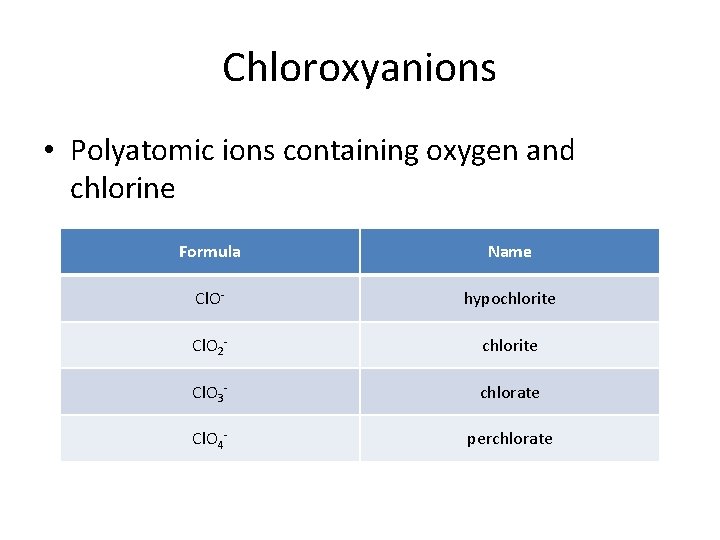
Chloroxyanions • Polyatomic ions containing oxygen and chlorine Formula Name Cl. O- hypochlorite Cl. O 2 - chlorite Cl. O 3 - chlorate Cl. O 4 - perchlorate

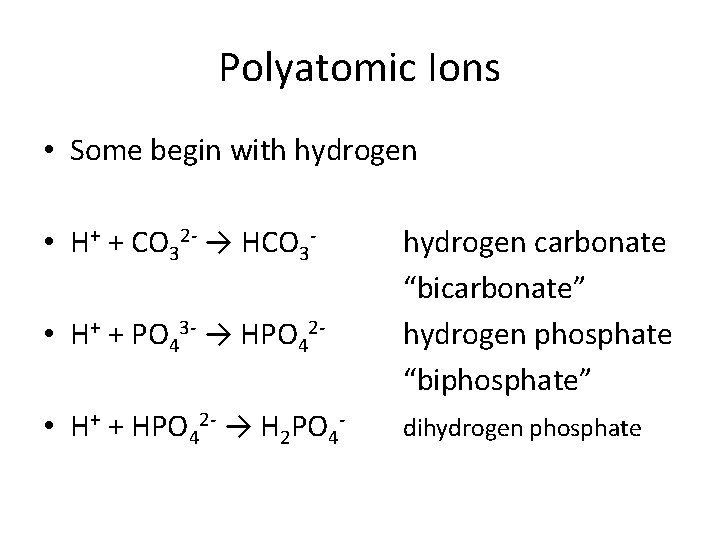
Polyatomic Ions • Some begin with hydrogen • H+ + CO 32 - → HCO 3 • H+ + PO 43 - → HPO 42 • H+ + HPO 42 - → H 2 PO 4 - hydrogen carbonate “bicarbonate” hydrogen phosphate “biphosphate” dihydrogen phosphate

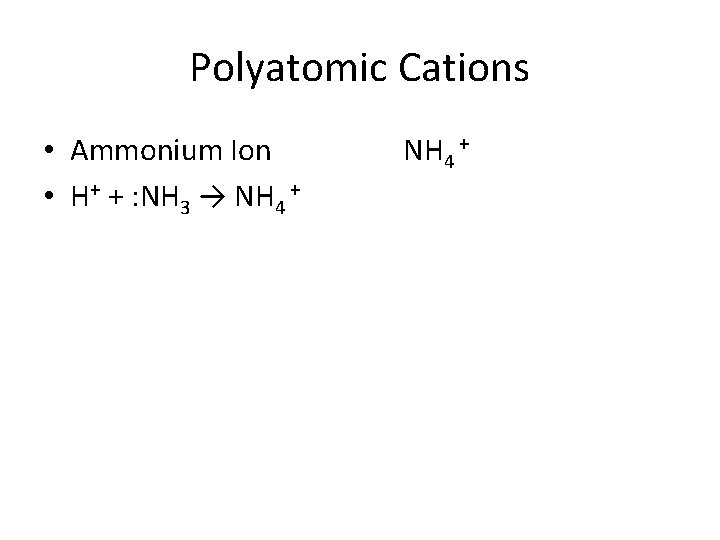
Polyatomic Cations • Ammonium Ion • H+ + : NH 3 → NH 4 +


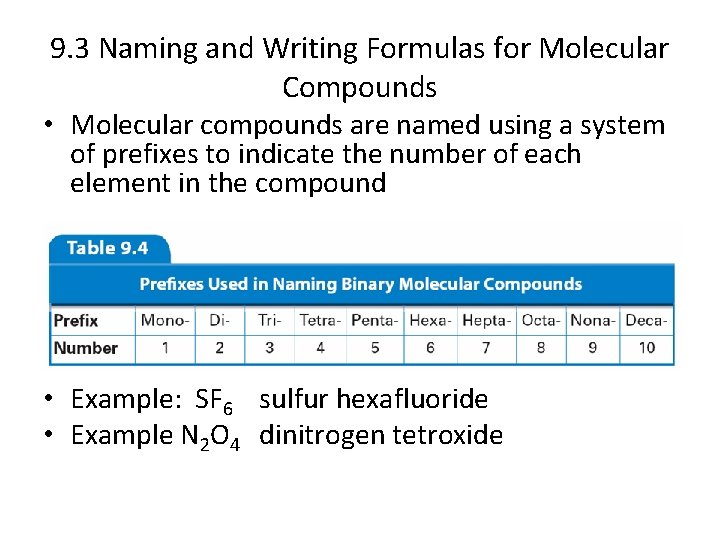
9. 3 Naming and Writing Formulas for Molecular Compounds • Molecular compounds are named using a system of prefixes to indicate the number of each element in the compound • Do not use mono- before the first element • Example: SF 6 sulfur hexafluoride • Example N 2 O 4 dinitrogen tetroxide


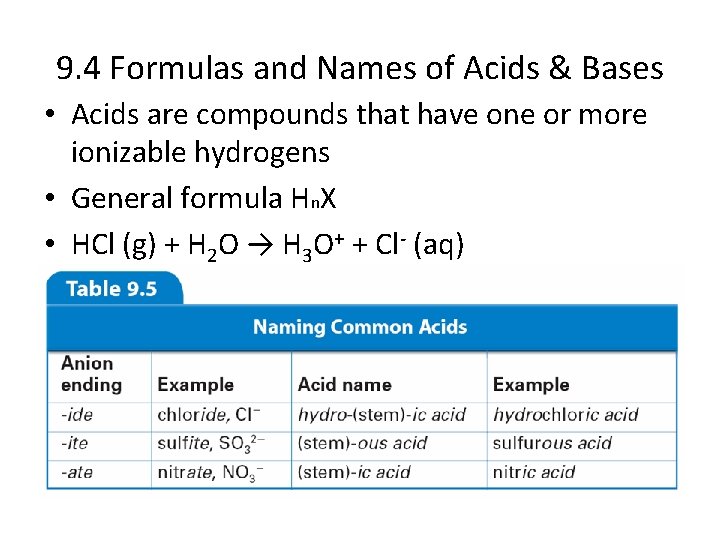
9. 4 Formulas and Names of Acids & Bases • Acids are compounds that have one or more ionizable hydrogens • General formula Hn. X • HCl (g) + H 2 O → H 3 O+ + Cl- (aq)

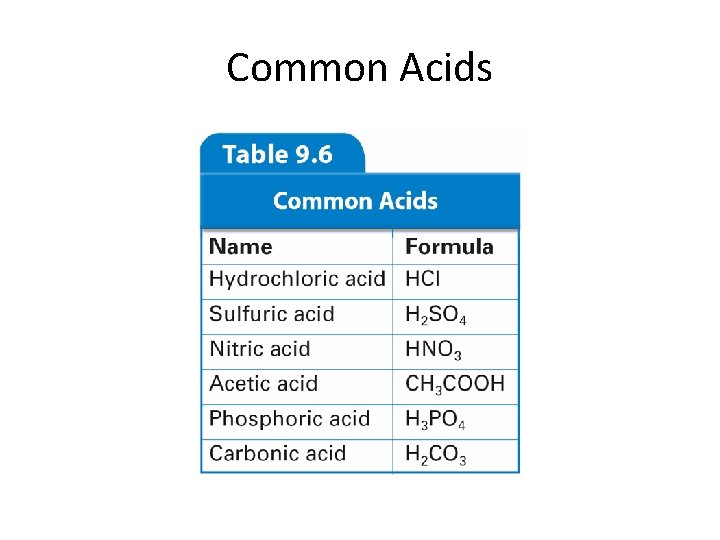
Common Acids


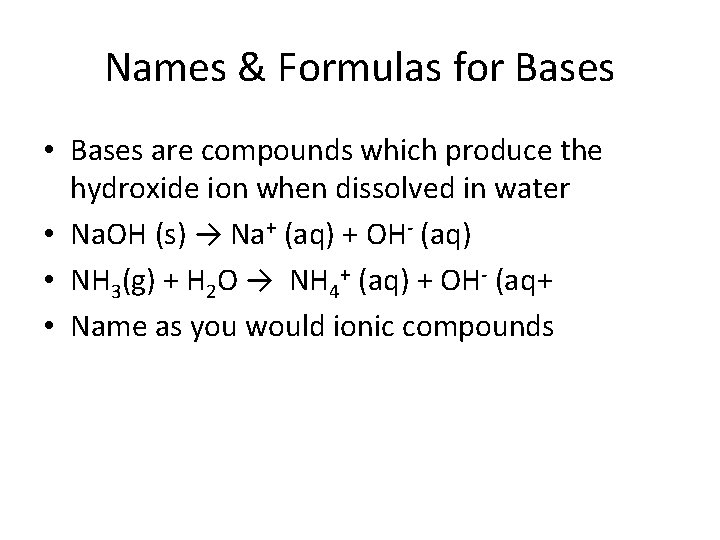
Names & Formulas for Bases • Bases are compounds which produce the hydroxide ion when dissolved in water • Na. OH (s) → Na+ (aq) + OH- (aq) • NH 3(g) + H 2 O → NH 4+ (aq) + OH- (aq+ • Name as you would ionic compounds

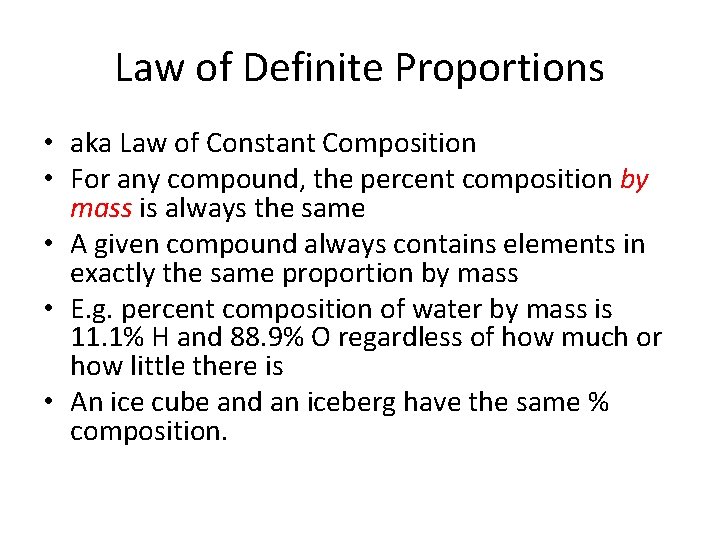
Law of Definite Proportions • aka Law of Constant Composition • For any compound, the percent composition by mass is always the same • A given compound always contains elements in exactly the same proportion by mass • E. g. percent composition of water by mass is 11. 1% H and 88. 9% O regardless of how much or how little there is • An ice cube and an iceberg have the same % composition.

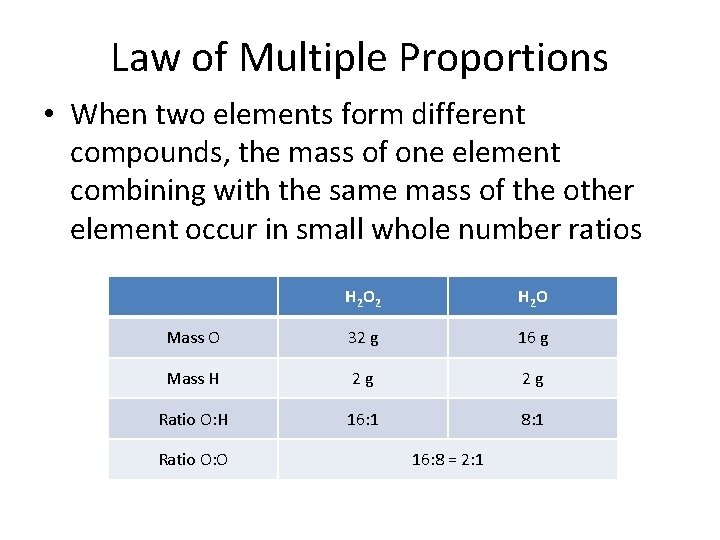
Law of Multiple Proportions • When two elements form different compounds, the mass of one element combining with the same mass of the other element occur in small whole number ratios H 2 O 2 H 2 O Mass O 32 g 16 g Mass H 2 g 2 g Ratio O: H 16: 1 8: 1 Ratio O: O 16: 8 = 2: 1

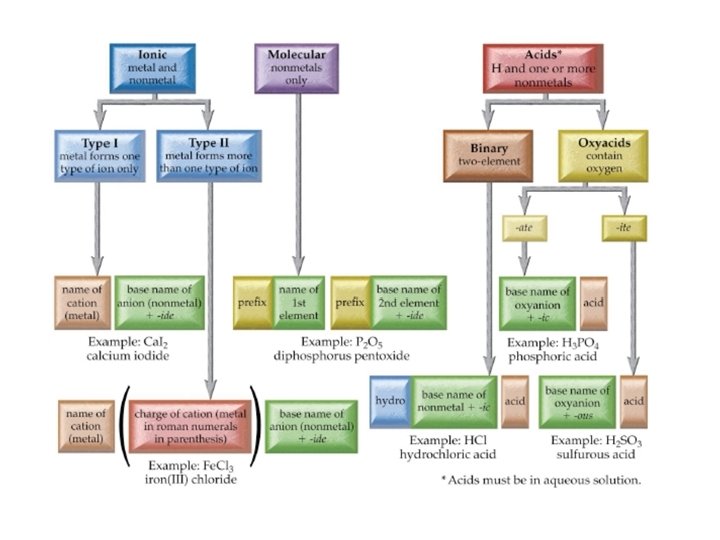
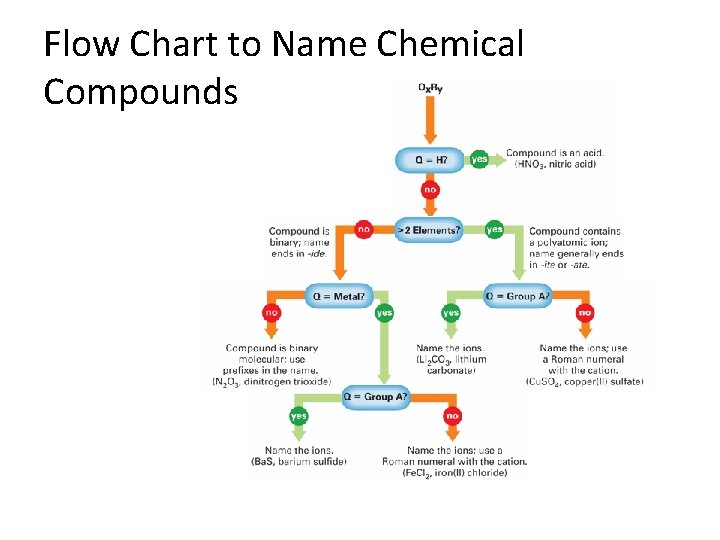
Flow Chart to Name Chemical Compounds