Chemical Names and Formulas Chemistry Mrs Chast Substance

Chemical Names and Formulas Chemistry Mrs. Chast
Substance Classification n. Every substance is either an element or a compound

Noble Gases n In nature, only noble gases tend to exist as isolated atoms. They are MONATOMIC – single atoms
A Compound n Consists of more than one kind of atom
A compound is either n Molecular nature or ionic in
I. Molecules and Molecular Compounds n. A molecule is the smallest electrically neutral unit of a substance that still has properties of the substance
I. Molecules and Molecular Compounds n Molecules are made up of one or more atoms that act as a unit n Diatomic molecules are composed of 2 atoms n Triatomic molecules are composed of 3 atoms
I. Molecules and Molecular Compounds n Atoms of different elements combine in whole-number ratios to form compounds
I. Molecules and Molecular Compounds n Compounds composed of covalent bonds are called molecular compounds

Molecular Compounds n Tend to have relatively low melting points and no conductivity
Most molecular compounds n Are composed of 2 or more nonmetals covalently bonded n The molecules of a given molecular compound are all the same

II. Ions and Ionic Compounds n Not all compounds are molecular. Many compounds are composed of particles called ions

II. Ions and Ionic Compounds n Ions are atoms or groups of atoms that have a positive or negative charge
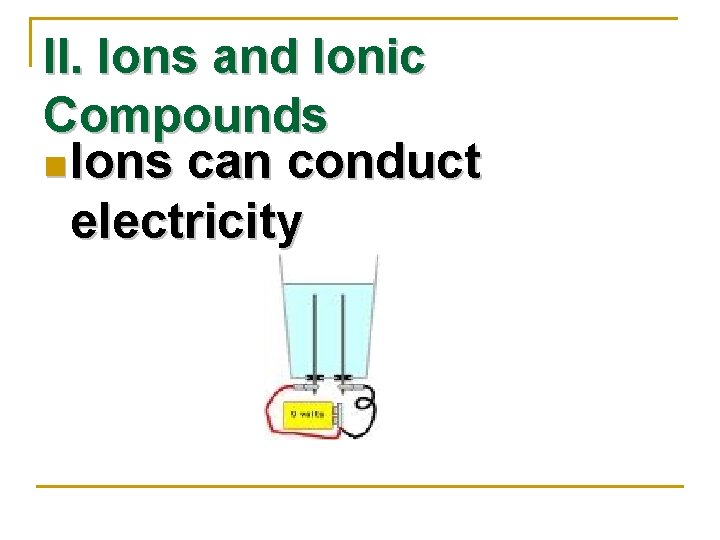
II. Ions and Ionic Compounds n Ions can conduct electricity
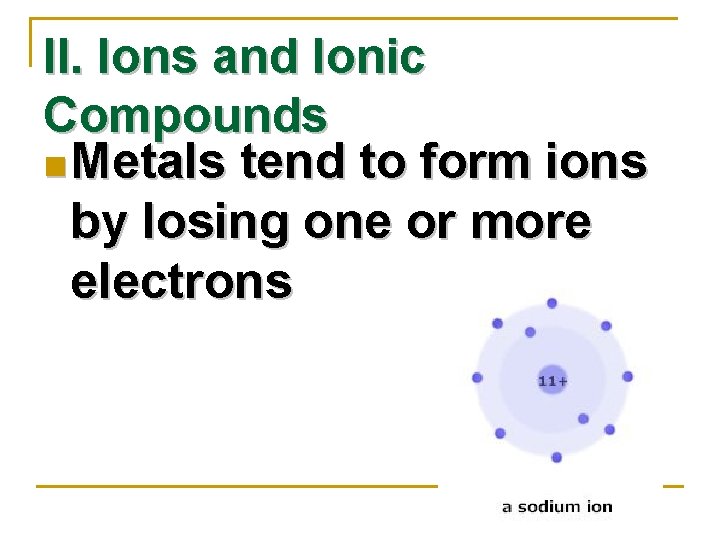
II. Ions and Ionic Compounds n Metals tend to form ions by losing one or more electrons

Cations! n. A cation is any atom or group of atoms that has a positive charge

Name that Cation n For metallic elements, the name of a cation is always the same as the name of the element
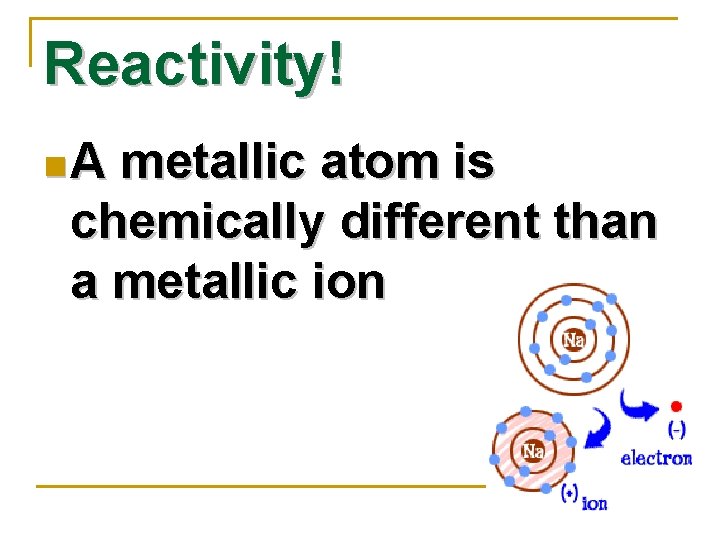
Reactivity! n. A metallic atom is chemically different than a metallic ion

Metals tend to form cations n By LOSING one or more electrons
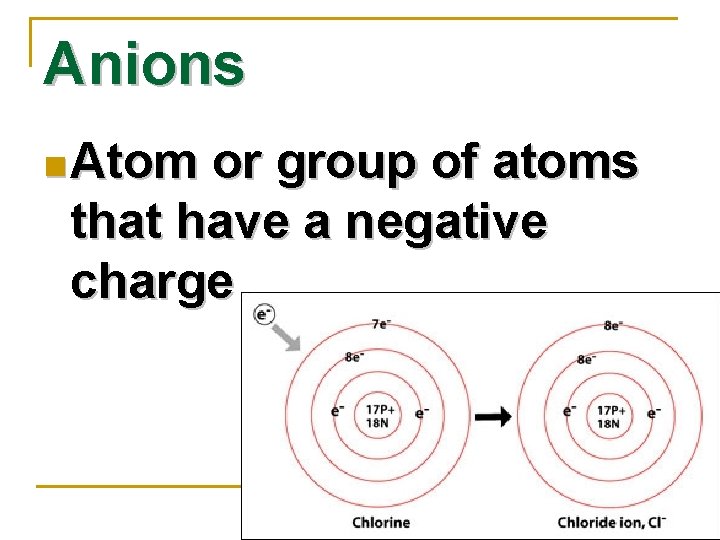
Anions n Atom or group of atoms that have a negative charge

Name Me n The name of an anion of a nonmetal is not the same as the element name. The name typically ends in -ide
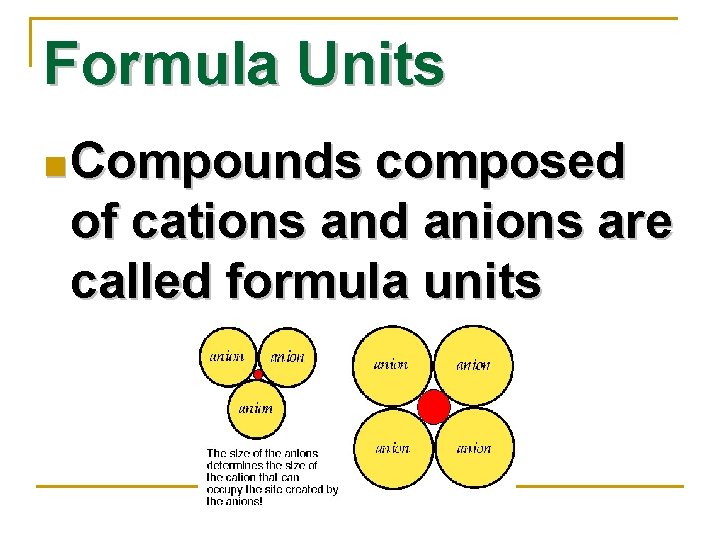
Formula Units n Compounds composed of cations and anions are called formula units
Overall Charge n Although they are composed of ions, ionic compounds are neutral! n The TOTAL positive charge = the total negative charge

Ionic compounds n Usually solid crystals at room temperature and melt at very high temperatures

III. Chemical Formulas n Shows the kinds and numbers of atoms in the simplest representative unit of the substance

Subscripts n If the molecule has more than one atom of the element, a subscript is used
IV. Molecular Formulas n The chemical formula of a molecular compound is called a molecular formula Molecular formulas show the composition of a molecule, NOT the structure

V. Formula Units n Formula units are used to represent an ionic compound n A formula unit is the simplest whole-number ratio of ions in the compound

Ionic charges n Are used in the crisscross method to derive the correct formula, but they are not shown in the formula
Law of Definite Proportions n In samples of any chemical compound, the mass of the elements are always in the same proportions
Law of Multiple Proportions n Whenever two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers
VII. Monatomic Ions n Ionic charge = oxidation number n Numerical charge is determined by the difference of the group number from 8. Sign of nonmetals is negative!

Noble Gases n DO NOT form ions!
Transition Metals n Many transition metals are capable of forming 2 cations. There are 2 ways to name them. n -ous is the lower of the charges n -ic is the higher of the charges

VII. Polyatomic Ions n Tightly bound atoms that behave as a unit and carry a charge n Most end in –ite or –ate n -ite is one less oxygen than -ate

VIII. Writing Formulas for Binary Ionic n Compounds composed of two Compounds elements are called binary compounds. n When writing the formula for a binary compound, the cation is always written first. n The positive charge = the negative charge. The net ionic charge is zero

Written Using Criss Cross Method n Remember to use the CHARGE of ions. n Ca+2 and S-2 would crisscross to Ca 2 S 2, but lowest whole number ratio of ions reduce to Ca. S
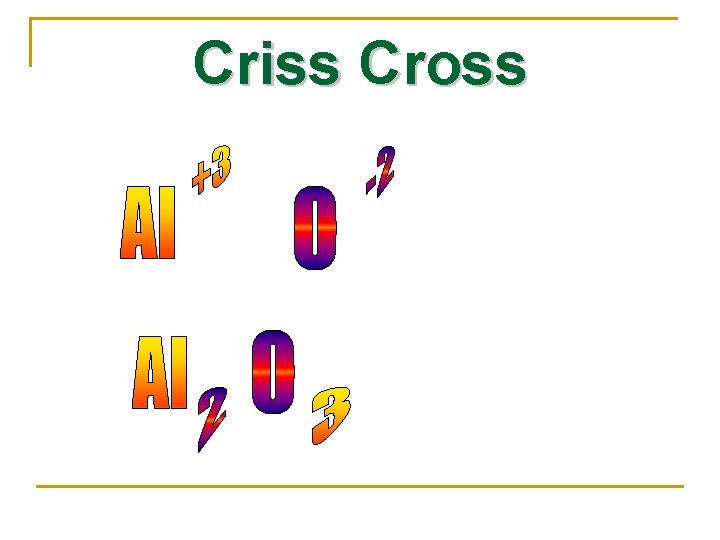
Criss Cross

IX. Naming Binary Ionic Compounds n Name the compound by naming the ions in the order written in the formula n The name of the transition metal that has more than one ionic charge must include a roman numeral

Ternary Ionic Compounds Ternary compounds contain three n n elements Write the formula for each ion in the order listed in the name, then use crisscross to determine subscripts If more than one polyatomic ion is needed, place the polyatomic ion formula in parenthesis, followed by a subscript When naming ternary compounds must recognize the polyatomic ions first.
XI. Binary Molecular Compounds n Composed of two nonmetals n They are composed of atoms NOT IONS, so criss cross does NOT work n Use greek prefixes to tell how many ATOMS are present
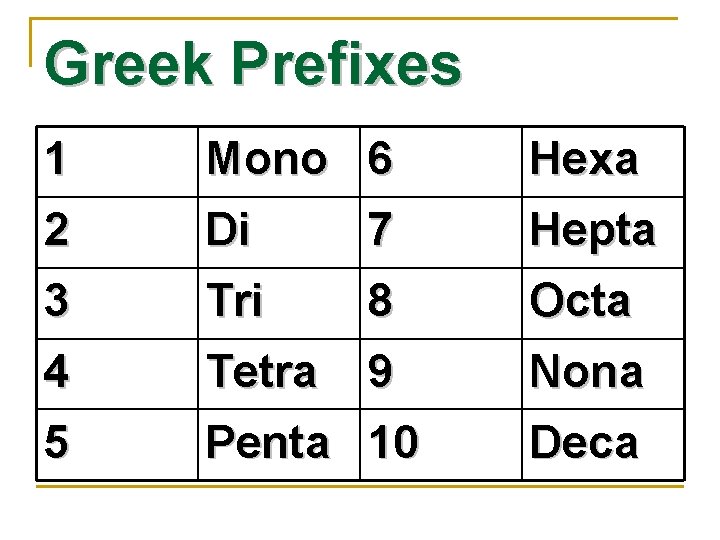
Greek Prefixes 1 Mono 6 Hexa 2 3 Di Tri Hepta Octa 4 5 Tetra 9 Penta 10 7 8 Nona Deca

Rules n Always end the name in –ide n The vowel at the end of a prefix is often dropped when the name of the element begins with a vowel n When a single atom of the first element, DO NOT WRITE mono
XII. Naming Common Acids n Consider acids to be combinations of anions connected to as many hydrogens as needed to make the molecule neutral

Binary Acids n Hydro ______ ic acid n Hydrochloric Acid (HCl) n Hydroiodidic Acid (HI)
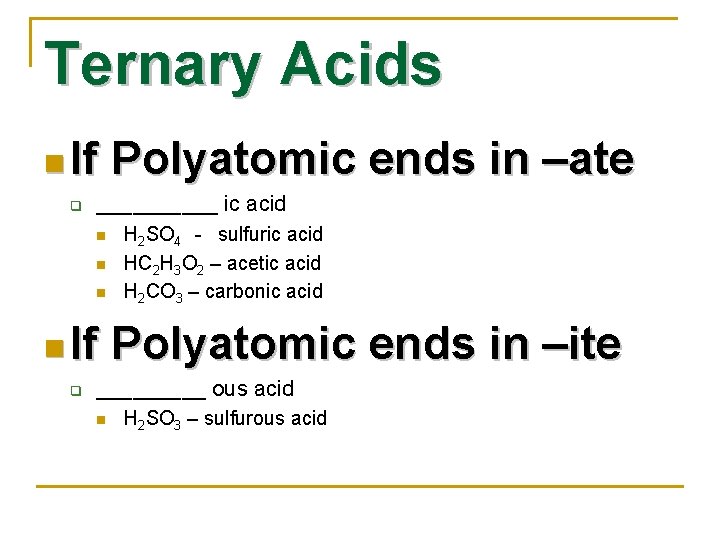
Ternary Acids n If q _____ ic acid n n If q Polyatomic ends in –ate H 2 SO 4 - sulfuric acid HC 2 H 3 O 2 – acetic acid H 2 CO 3 – carbonic acid Polyatomic ends in –ite _____ ous acid n H 2 SO 3 – sulfurous acid
- Slides: 46